摘要/Abstract
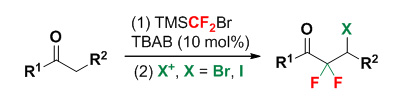
以(溴二氟甲基)三甲基硅烷(TMSCF2Br)为二氟卡宾源,利用其兼具TMS转移特性及自活化特性,仅在催化量的n-Bu4NBr的催化启动下,直接完成甲基酮的烯醇化-二氟环丙烷化反应以及与卤正离子的开环卤化反应,并使用卤负离子作为卤源,采用其原位氧化形成卤正离子的方法实现了偕二氟环丙烷硅醚的开环-卤化,合成了β-卤代-α,α-二氟代酮类物质.
关键词: 酮, α,α-二氟代酮, 偕二氟环丙烷硅醚, 多米诺反应
α,α-Difluoroketones represent an important subclass of organofluorine compounds, and have been widely applied in medicinal chemistry, particularly as enzyme inhibitors. Efficient use of organofluorine reagents plays a key role for the synthesis of fluorine-containing organic compounds. As an environmental and efficient difluorocarbene reagent, TMSCF2Br has been well utilized in synthetic applications. In 2013, Hu first utilized TMSCF2Br as a general difluorocarbene source for the difluoromethylenation of alkenes/alkynes as well as the difluoromethylation of O-, S-, N-, and P-nucleophiles. Moreover, Dilman realized the rapid assembly of various CF2-containing products by using TMSCF2Br as a difluorocarbene source, which depended on the concept of three independent components: difluorocarbene, nucleophile, and electrophile. Compared with the previous works, we recently reported a catalytic difluorocyclopropanation of enolizable ketones by using TMSCF2Br reagent, which acts as not only the difluorocarbene source but also the TMS transfer agent. The in situ generated siloxydifluorocyclopropanes were used for the synthesis of α-fluoroenones, o-fluoronaphthols, α,α-difluorocyclopentenones and α,α-difluorocyclopentanones compounds. Here, we report a simple and effective method for the conversion of enolizable ketones to α,α-difluoro-β-halo-substituted ketones. The whole process involves the in situ formation and regioselective ring opening halogenation of siloxydifluorocyclopropanes. The reaction features easily available raw materials, simple operation and practical method. A representative procedure for this reaction is as following: To a dried polytetrafluoroethene (PTFE) sealed pressure tube were added ketone 1 (0.5 mmol), n-Bu4NBr (0.05 mmol, 10 mol%), TMSCF2Br (0.75 mmol) and toluene (2.5 mL) in sequence. The reaction mixture was stirred at 110 ℃ for 2 h, followed by adding an additional amount of TMSCF2Br (0.5 mmol) for another 4 h. Removal of toluene under reduced pressure delivered a mixture mainly containing 2. The reaction system was allowed to cool to room temperature followed by adding NBS/NIS (0.75 mmol) and CH3CN (2 mL). The resulting mixture was stirred at room temperature for 2 h to consume 2 and then poured into saturated NaCl solution (30 mL), extracted with CH2Cl2 (10 mL×3). The combined organic extracts were dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to yield the crude product, which was purified by silica gel chromatography (petroleum ether/ethyl acetate: 100/1, V/V) to afford the pure product 4/5.
Key words: ketone, α,α-difluoroketone, siloxydifluorocyclopropane, domino reaction
PDF全文下载地址:
点我下载PDF
