摘要/Abstract
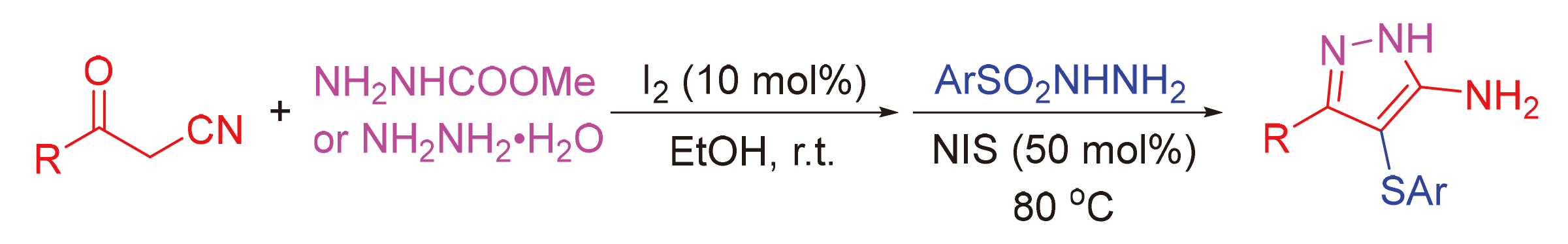
建立了3-氧代-3-芳基丙腈、肼基甲酸甲酯(或水合肼)和芳基磺酰肼的一锅两步反应. 在I2和N-碘代丁二酰亚胺(NIS)的作用下, 通过环化、磺基化和脱酯基化反应构建了一系列3-芳基-4-(芳硫基)-1H-吡唑-5-胺化合物. 该方法具有良好的原子经济性、温和的反应条件、广泛的底物适用范围和克级规模的合成. 此外, 还对3-芳基-4-(芳硫基)-1H-吡唑-5-胺产物的进一步转化进行了研究.
关键词: 一锅两步, 1H-吡唑-5-胺, 环化, 亚磺酰化
One-pot two-step reaction of 3-oxo-3-arylpropanenitriles, methyl hydrazinecarboxylate (or hydrazine hydrate), and arylsulfonyl hydrazides has been established, and a series of 3-aryl-4-(arylthio)-1H-pyrazol-5-amines were constructed by sequential cyclization, sulfenylation, and removal of COOMe group under the action of I2 and N-iodo-succininide (NIS), respectively. The method represents good atomic economy, mild reaction conditions, broad substrate scope, and gram-scale synthesis. Moreover, the further transformations of 3-aryl-4-(arylthio)-1H-pyrazol-5-amine products were also investigated.
Key words: one-pot two-step, 1H-pyrazol-5-amine, cyclization, sulfenylation
PDF全文下载地址:
点我下载PDF
