摘要/Abstract
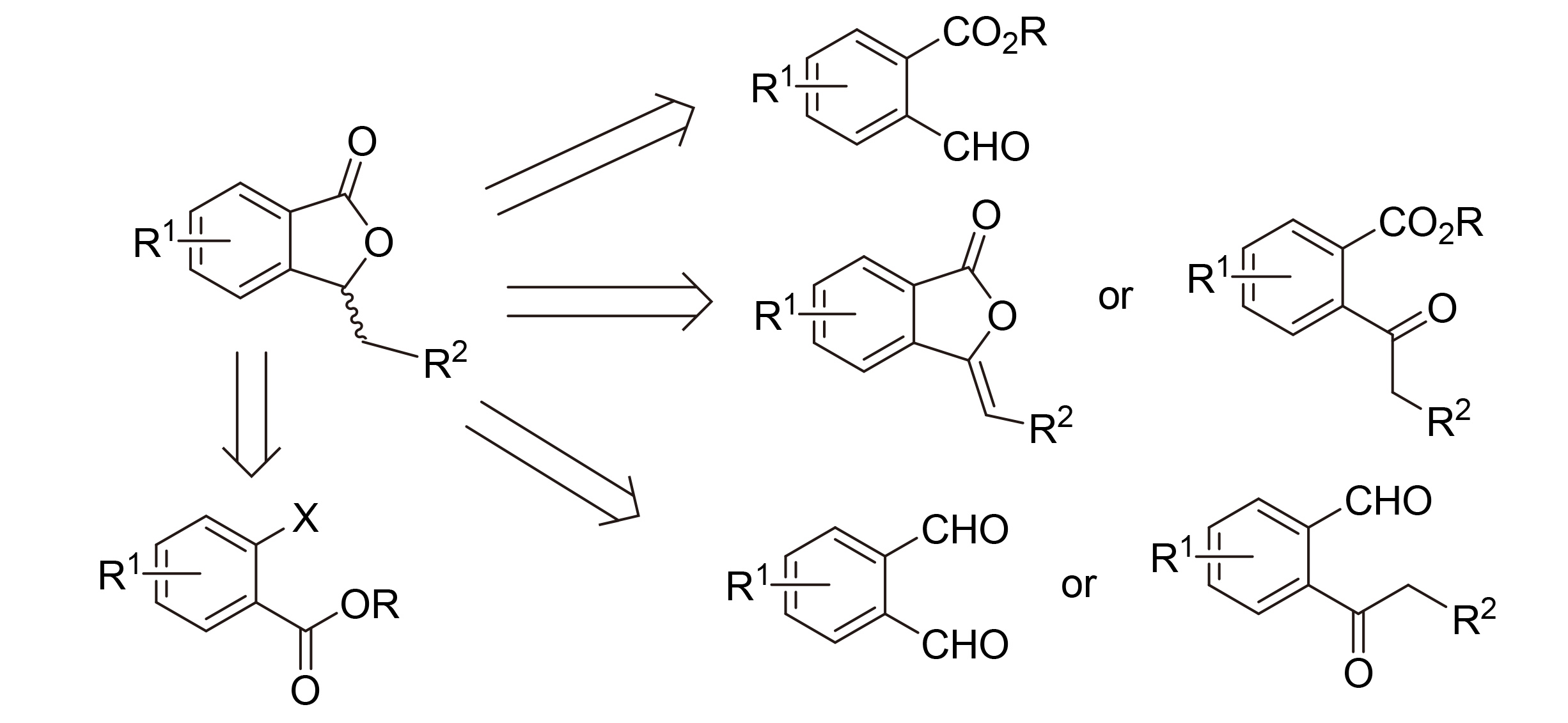
含3-取代苯酞结构的化合物广泛存在于植物和真菌中, 是传统中草药中的活性成分, 在现代医药中受到广泛关注. 本综述列举了部分具有生物活性的苯酞类化合物, 综述了3-取代苯酞类化合物的合成研究进展, 特别是近年来报道的对映体选择性合成方法, 以期对设计、发现具有广泛适用性和高立体选择性地合成苯酞骨架或类似化合物的方法产生启示作用. 这些方法包括: (a)通过形成C—C键的反应构建内酯, 例如2-酰基苯甲酸酯等的醇醛缩合/内酯化级联反应; (b)通过形成C—O键的反应构建内酯, 例如2-酰基苯甲酸酯的还原内酯化或3-烯基苯酞的还原, 分子内氧化/内酯化, 或分子内氧化还原/内酯化. 这些方法对于高立体选择性合成苯酞类化合物和药物研究具有重要意义.
关键词: 苯酞, 异苯并呋喃-1(3H)-酮, 合成, 对映选择性, 天然产物
3-Substituted phthalides are widely distributed in plants and fungi. They are active ingredients in traditional Chinese herbal medicines, and have attracted much attention in modern medicinal chemistry. The synthetic methods of 3-substituted phthalides are reviewed, especially those in enantioselective manners. The main approach involves: (a) construction of lactones from C—C bond formation reactions, e.g. an aldol/lactonization cascade reaction of 2-acylbenzoates and alikes, (b) construction of lactones via C—O bond formation reactions, e.g. reductive lactonization of 2-acylbenzoates or reduction of 3-alkenyl phthalides, intramolecular oxidation/lactonization, or intramolecular redox/lactonization. These methods are of great significance for the high stereoselective synthesis of phthalides and drug research.
Key words: phthalide, isobenzofuran-1(3H)-one, synthesis, enantioselectivity, natural product
PDF全文下载地址:
点我下载PDF
