摘要/Abstract
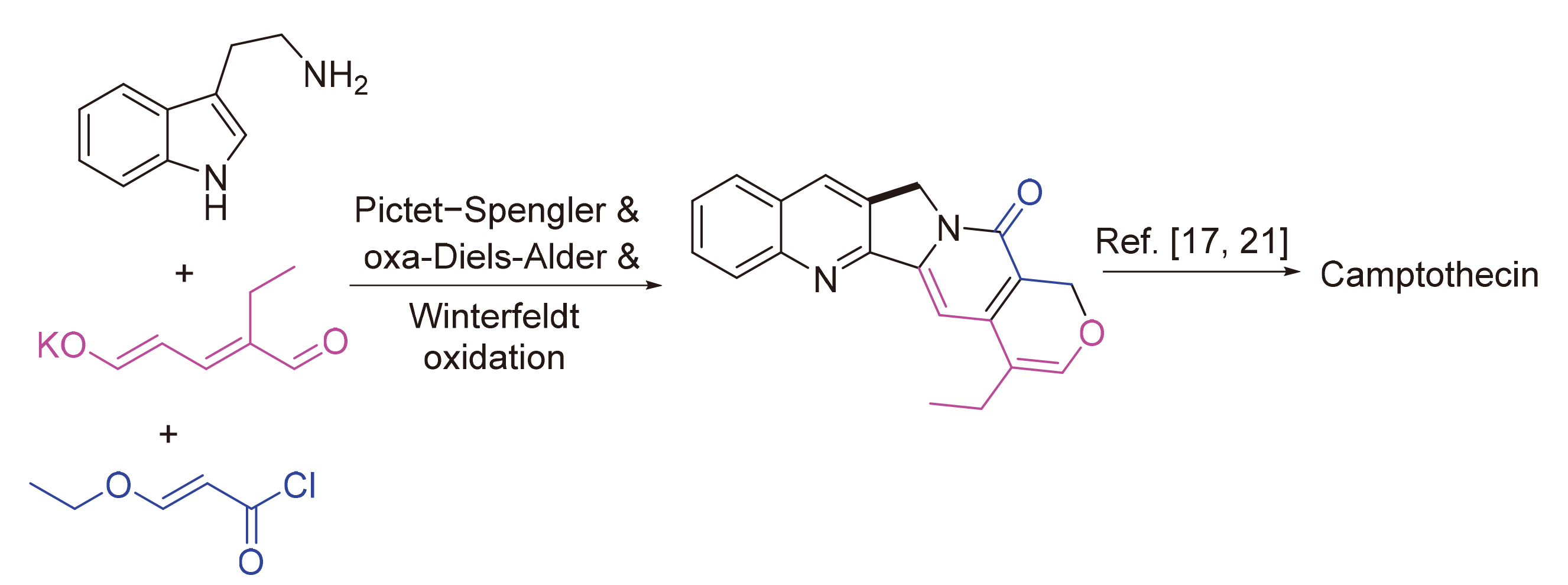
以色胺和方便易得的乙基戊烯二醛盐为起始物, 以简洁的步骤(最终实现喜树碱全合成共9步反应)完成了喜树碱的生源启发下的无保护基形式合成. 合成路线涉及关键的Pictet-Spengler反应, 分子内氧杂Diels-Alder反应高效构建单萜吲哚中间体, 以及Winterfeldt高效仿生氧化吲哚合成喹啉酮结构.
关键词: 喜树碱, Pictet-Spengler反应, 氧杂Diels-Alder反应, Winterfeldt氧化, 形式合成
A concise biogenetically inspired formal synthesis of camptothecin without use of protecting groups has been developed starting from tryptamine and easily prepared ethyl glutaconaldehyde salt. The synthesis features the key Pictet-Spengler reaction, efficient intramolecular oxa-Diels-Alder reaction to construct heptacyclic monoterpenoid indole alkaloid intermediate, as well as the following Winterfeldt biomimetic oxidation to form quinolinone moiety from indole skeleton.
Key words: Camptothecin, Pictet-Spengler reaction, oxa-Diels-Alder reaction, Winterfeldt oxidation, formal synthesis
PDF全文下载地址:
点我下载PDF
