摘要/Abstract
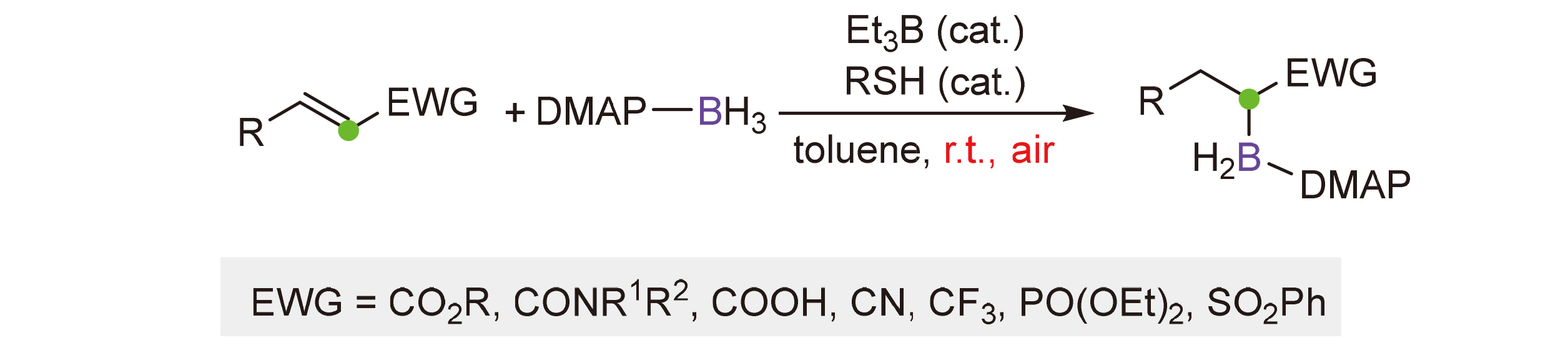
含硼有机化合物在合成化学中具有重要的应用价值, 硼氢化反应是构建有机硼化合物最常用的方法之一. 利用4-二甲氨基吡啶硼自由基与缺电子烯烃的自由基硼氢化反应, 高区域选择性地构建了α-硼取代产物. 该反应条件温和, 官能团容忍性好, 底物范围广. 多种α,β-不饱和酯、酰胺、羧酸、腈、三氟甲基化合物、砜以及磷酸酯均能顺利发生反应, 得到的含硼产物可以进一步应用于后续碳-碳键的构建.
关键词: 4-二甲氨基吡啶硼自由基, 缺电子烯烃, 区域选择性, 硼氢化反应, 自由基化学
Organoboron compounds have shown significant applications in modern chemical synthesis. Hydroboration of alkenes is among the most widely used methods to access these targets. Herein, a regioselective radical hydroboration reaction of electron-deficient alkenes with 4-dimethylaminopyridine (DMAP)-boryl radical for the synthesis of α-boryl functionalized molecules is reported. The reaction features specific α-regioselectivity, mild reaction conditions, good functional group tolerance, and broad substrate scope. α,β-Unsaturated esters, amides, carboxylic acid, nitrile, trifluoromethyl molecule, sulfone, and phosphonate are viable substrates for this reaction. The resulting α-boryl functionalized molecules can be further transformed to various useful building blocks.
Key words: 4-dimethylaminopyridine-boryl radical, electron-deficient alkenes, regioselectivity, hydroboration, radical chemistry
PDF全文下载地址:
点我下载PDF
