摘要/Abstract
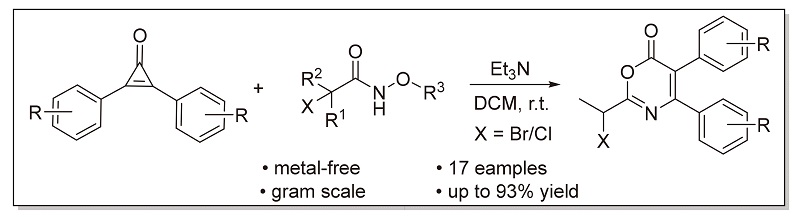
利用环丙烯酮同时具有亲核性、亲电性以及易发生开环反应的特点, 实现了三乙胺促进的环丙烯酮和α-卤代异羟肟酸酯类化合物的[3+3]环加成反应, 快速构筑了6H-1,3-噁嗪-6-酮骨架, 为噁嗪酮类化合物的合成提供了新的思路. 该反应在无金属和温和条件下显示出良好的收率和官能团耐受性, 同时适合克级规模制备.
关键词: 环丙烯酮, α-卤代异羟肟酸酯, 环化反应, 噁嗪酮
A triethyl amine-promoted cyclization reaction between cyclopropenone and α-halohydroxamate has been developed to give an alternative synthetic strategy for the construction of 6H-1,3-oxazin-6-one skeleton. The reaction shows good yield and functional group tolerance under metal-free and mild conditions, and it is suitable for gram-scale preparation.
Key words: cyclopropenone, α-halogenated hydroxamate, cyclization reaction, oxazinone
PDF全文下载地址:
点我下载PDF
