摘要/Abstract
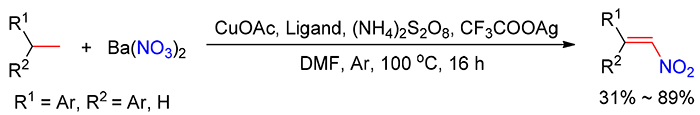
硝基烯烃是有机合成化学中常见的重要中间体, 其合成方法主要通过硝基烷烃与醛或酮的缩合、消除, 烯烃直接脱氢硝化或者烯基羧酸脱羧硝化得到目标产物, 但是这些合成方法由于原料价格昂贵, 在大规模生产中受到限制. 本研究首次采用廉价易得的芳基乙烷与硝酸钡为原料, 以铜/银为催化剂, 过硫酸钾为氧化剂, 通过脱氢硝化反应合成硝基芳香烯烃. 在优化的反应体系中, 1,1-二苯基乙烷、苯基乙烷、4-乙基联苯及乙基萘类化合物能与硝酸钡进行脱氢硝化反应, 以中等至好的收率获得 E型硝基芳香烯烃.
关键词: 硝基芳香烯烃, 芳基乙烷, 硝酸钡, 脱氢硝化, 过硫酸铵
Nitroolefin is a common and versatile reagent, synthesis of which from aldehydes/ketones, α, β-unsaturated carboxylic acids or olefins is generally limited by the high cost of raw materials in industrial processes in the future. Herein, an alternative and economical protocol for the synthesis of nitroaromatic olefins directly from easily available arylethanes with barium nitrate using Cu/Ag as cocatalyst and ammonium persulfate as the terminal oxidant is reported. Additionally, 1,1-diphenylethanes, phenylethanes, 4-ethyl-1,1'-biphenyl and ethylnaphthalenes were suitable substrates for the current dehydrogenative nitration, and provided E-nitroaromatic olefins in moderate to good yields.
Key words: nitroaromatic olefins, arylethanes, barium nitrate, dehydrogenative nitration, ammonium persulfate
PDF全文下载地址:
点我下载PDF
