摘要/Abstract
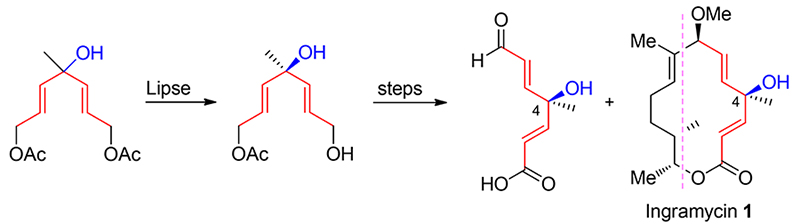
在构筑天然产物英格拉霉素分子中的手性叔醇时, 不采用常规的不对称合成及使用手性源的方法, 利用分子本身结构上的对称性, 首先合成对称的非手性前体化合物, 然后在脂肪酶催化下进行选择性酯水解反应, 既推进了官能团的转化, 又构筑了合成上具有挑战性、位阻较大的季碳立体中心. 本路线以易得的烯丙基溴和乙酸乙酯为原料, 经11步反应合成了英格拉霉素右部片断, 总收率26.7%, ee值为50.84%.
关键词: 英格拉霉素, 去对称化, 脂肪酶, 手性叔醇
In the construction of chiral tertiary alcohol of the natural product ingramycin, the conventional asymmetric synthesis and the method that using the chiral source as material were not used. Instead, exploring the symmetry of natural product itself, the symmetric nonchiral precursor was synthesized firstly, and then the selective ester hydrolysis reaction under lipase catalysis was carried out, which not only promoted the transformation of functional groups, but also constructed a challenging and sterically congested quaternary carbon stereocenter. Based on the readily available allyl bromide and ethyl acetate, the right segment of ingramycin was synthesized by 11 steps with total yield of 26.7% and ee value of 50.84%.
Key words: ingramycin, desymmetrization, lipase, chiral tertiary alcohol
PDF全文下载地址:
点我下载PDF
