摘要/Abstract
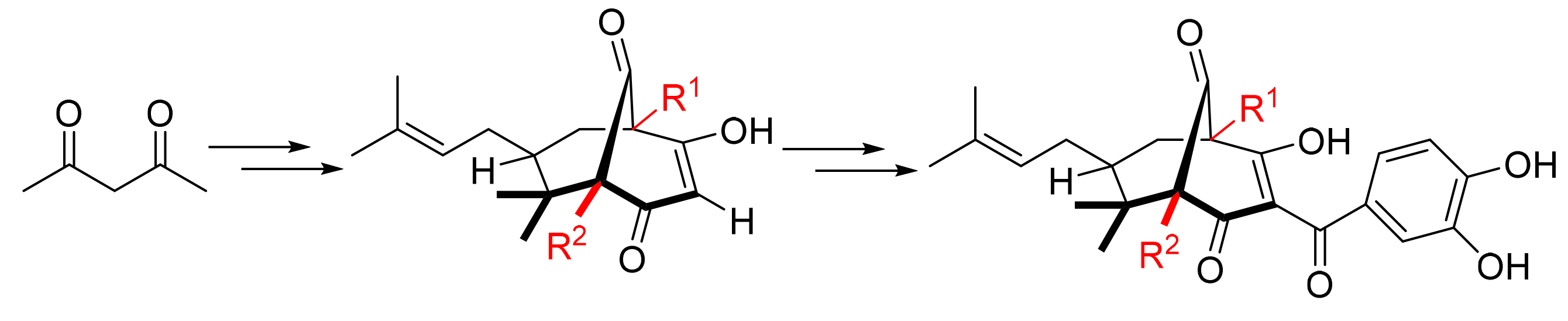
山竹醇具有广泛的生物学活性,例如抗炎、抗肿瘤、抗氧化、诱导细胞凋亡等.通过对山竹醇进行结构修饰,研究其侧链基团对山竹醇活性的影响,以期提高其抗肿瘤活性.以乙酰丙酮为原料,经过Michael加成、Knoevenagel缩合等多步反应,合成了三个未见文献报道的山竹醇类似物,并用噻唑蓝(MTT)法对其进行生物活性研究,分析其构效关系.结果表明,合成的三种新类似物对口腔鳞癌细胞的抑制活性均低于山竹醇,由此可知,C4位置的异戊烯基和C8位置的烯丙基对山竹醇的生物活性起关键作用.
关键词: 山竹醇, 结构修饰, Michael加成, 抗肿瘤
Garcinol possesses a wide range of biological activities, such as anti-inflammation, anti-tumors, anti-oxidation, induction of apoptosis and so on. In this paper, the modification of the side chains in garcinol was carried out to enhance its anti-tumor activity. Employing acetylacetone as starting material, three new analogs of garcinol were prepared by means of Michael addition, Knoevenagel condensation, and so on. Furthemore, their biological activity was assessed by methyl thiazolyl tetrazolium (MTT) method and the structure-activity relationship was analyzed. The results showed that the inhibitory activities of the three new analogs on oral squamous cell carcinoma were moderately lower than that of garcinol. Hence, the isoprenyl group at the C4 position and the allylic group at the C8 position might play vital roles for the biological activity of garcinol.
Key words: garcinol, structural modification, Michael addition, anti-tumor
PDF全文下载地址:
点我下载PDF
