摘要/Abstract
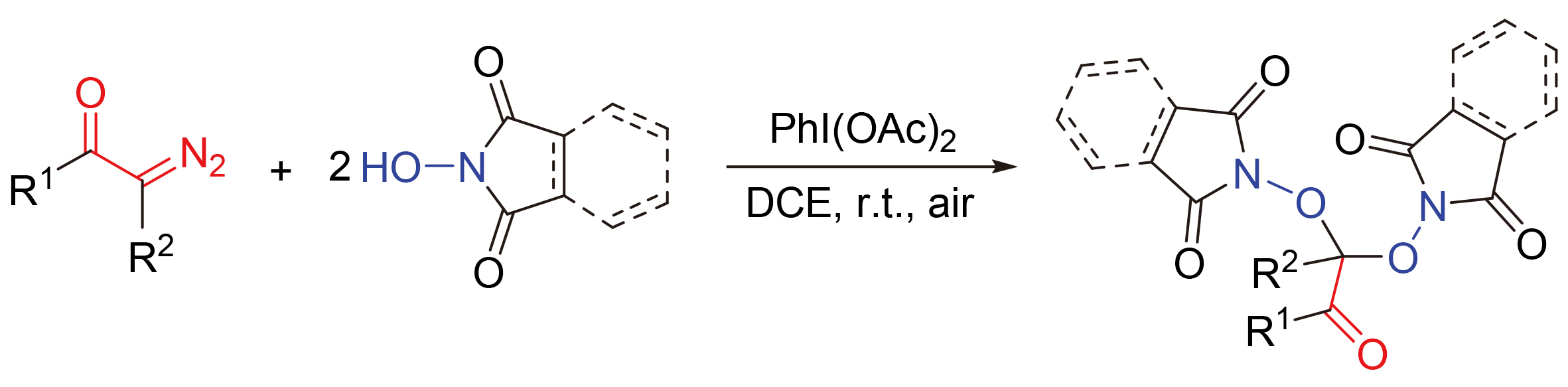
报道了一类新颖的醋酸碘苯介导的α-重氮羰基化合物的去重氮双氧合反应.该反应利用醋酸碘苯与N-羟基邻苯二甲酰亚胺(或N-羟基丁二甲酰亚胺)反应能产生氧中心自由基的特性实现了氧中心自由基诱导的α-重氮羰基化合物的双氧合反应,合成了一系列α,α-双氧代芳酮和α,α-双氧代羧酸酯衍生物,产率中等到良好.基于实验结果及文献报道,提出了可能的反应机理,其涉及氧中心自由基加成、C-N键的均裂和自由基交叉偶联等.此外,该反应具有无需金属催化剂、条件温和、操作简便等优点.
关键词: 双氧合反应, 去重氮化反应, α,α-双氧代芳酮, α,α-双氧代羧酸酯
A new PhI(OAc)2-mediated dediazodioxygenation of α-diazo carbonyls was reported. By using the characteristics of the in-situ-generated O-centered radicals from the interaction of PhI(OAc)2 and N-hydroxy phthalimide (or N-hydroxy succinimide), O-centered radical-triggered dioxygenation of α-diazo carbonyls was achieved in this transformation, which led to the synthesis of a series of α,α-dioxoarylketones and α,α-dioxoesters with moderate to good yields. Based on the experimental results and literature reports, the possible reaction mechanism was proposed, which involved O-centered radical addition, C-N bond homolysis and radical cross coupling. In addition, the reaction featured mild conditions and simple operation without any catalyst.
Key words: dioxygenation, dediazotization, α,α-dioxoarylketones, α,α-dioxoesters
PDF全文下载地址:
点我下载PDF
