摘要/Abstract
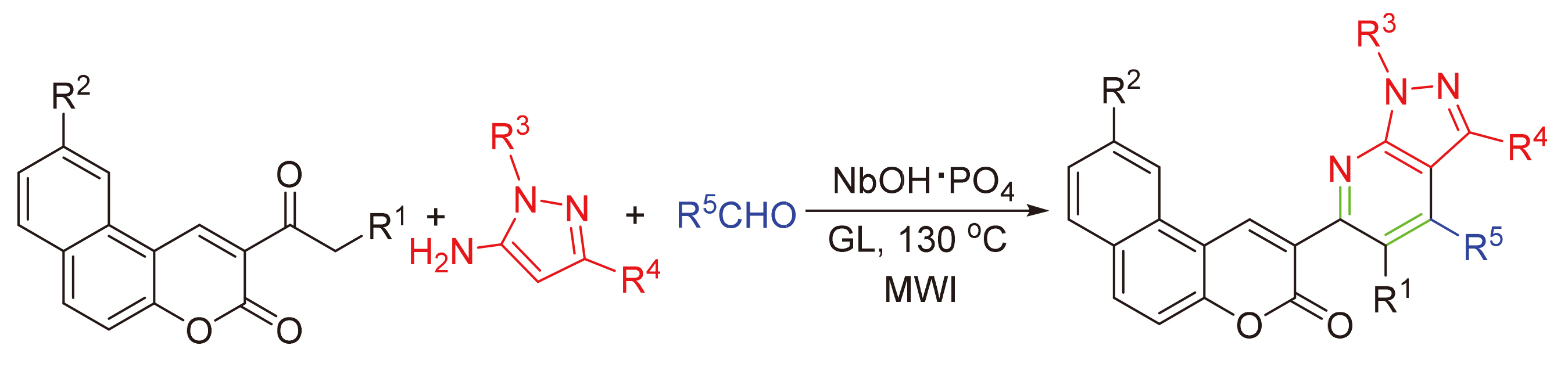
香豆素和吡唑并[3,4-b]吡啶骨架广泛存在于具有生物活性的天然化合物中,在药物化学中也被广泛用作药物核心单元,具有极其重要的作用.以磷酸改性铌酸作为催化剂,通过微波辐射下醛、香豆素衍生物、5-氨基吡唑的三组分反应一锅法高产率地合成一系列香豆素修饰的吡唑并[3,4-b]吡啶衍生物.该反应一步完成,具有催化剂和溶剂对环境友好,操作简单等优点.产物的结构经红外光谱、核磁共振谱及高分辨质谱予以确定.
关键词: 香豆素, 吡唑并[3,4-b]吡啶, 合成, 铌酸
Coumarin and pyrazolo[3,4-b]pyridine are structurally essential elements in biologically active natural compounds and are extremely important in medicinal chemistry by serving as key pharmacophores in drug discovery. In this article, the efficient synthesis of coumarin-fused pyrazolo[3,4-b]pyridine via three-component domino reaction of aldehydes, coumarin derivative and 5-aminopyrazole in one step catalyzed by niobic acid modified with phosphoric acid under microwave irradiation has been achieved. The one-pot procedure, eco-friendly catalyst and solvent as well as simple operation are the key features of this method. The structures of the products were identified by IR, NMR, and HRMS spectra.
Key words: coumarin, pyrazolo[3,4-b]pyridine, synthesis, niobic acid
PDF全文下载地址:
点我下载PDF
