摘要/Abstract
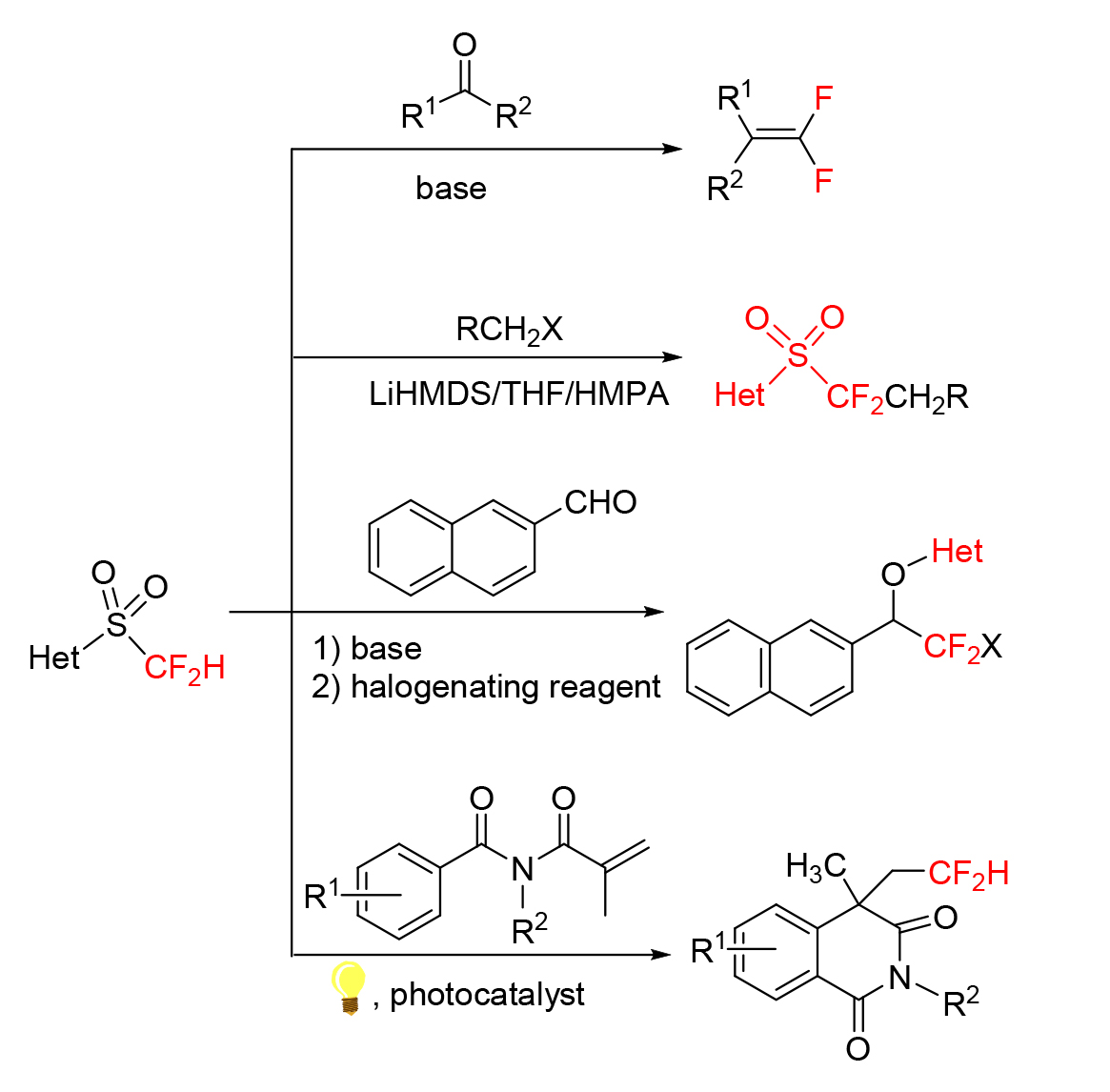
氟原子以及C—F键的独特性使得二氟亚甲基具有特殊的性质, 作为氧原子或羰基的生物电子等排体, 其在医药、农药与材料中起着有别于其他氟烷基的重要作用. 以二氟甲基2-吡啶基砜(胡试剂)为代表的二氟甲基杂芳基砜, 是近几年开发的新型二氟烷基化试剂, 因其具有易制备、官能团耐受性良好及对多种羰基化合物具有普遍适用性等优点而广受合成化学工作者的关注. 该类含氟试剂主要通过亲核取代反应、亲核加成反应、Julia-Kocienski烯化反应和自由基介导的双官能团化等反应, 将二氟甲基、二氟亚甲基、二氟烯基及其他二氟烷基引入醛、酮和杂环化合物的结构中. 首次从反应类型及其应用研究的角度综述了近十年来各种二氟甲基杂芳基砜参与的含氟有机化合物的合成研究.
关键词: 二氟甲基杂芳基砜, 亲核反应, Julia-Kocienski烯化反应, 自由基双官能团化反应
Due to the uniqueness of fluorine atom and C—F bond, difluoromethylene has special properties. As a bioisostere of an oxygen or a carbonyl group, it plays an important role in medicines, pesticides and materials. Difluoromethyl heteroaryl sulfones, represented by 2-PySO2CF2H (Hu reagent), have been developed recently as difluoromethylation reagents, and widely recognized by synthetic chemists for their ease of preparation, good functional group tolerance and universal applicability to a wide range of carbonyl compounds. Through different types of reactions such as nucleophilic substitution, nucleophilic addition, Julia-Kocienski olefination reaction, and radical-mediated difunctionalization, they introduced difluoromethyl, difluoromethylene, gem-difluoroalkene and other fluorine-containing groups into aldehydes, ketones, and heterocyclic compounds. For the first time, the synthesis of fluorine-containing organic compounds involved in various difluoromethyl heteroaryl sulfones in the past decade is reviewed from the perspective of reaction types and their application studies.
Key words: difluoromethyl heteroaryl sulfones, nucleophilic reaction, Julia-Kocienski olefination, radical-mediated difunctionalization
PDF全文下载地址:
点我下载PDF
