摘要/Abstract
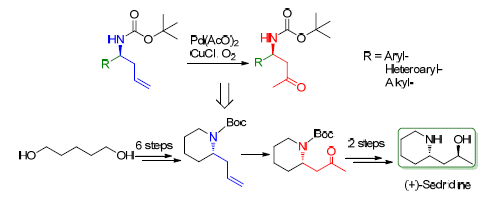
β-氨基羰基结构广泛存在于具有生理活性的小分子及药物中,并且是重要的合成砌块,可方便地转化为其它结构和片段.报道了一种新的制备β-氨基羰基化合物的方法.该方法使用氧气为氧化剂,醋酸钯、氯化亚铜为催化剂,可将手性高烯丙基胺类化合物转化为相应的β-氨基羰基化合物.该方法产率良好,底物适应性好,不影响分子中已有的手性中心.利用该方法完成了天然产物分子(+)-Sedridine的全合成工作,总收率32%.
关键词: β-氨基羰基化合物, 手性高烯丙基胺, Wacker反应, (+)-Sedridine
β-Amino-carbonyl fragments are important structures of many bioactive molecules and pharmaceuticals and critical synthetic blocks which can be easily converted into other structures and compounds. A novel method for preparing β-amino-carbonyl compounds with oxygen as an oxidant, palladium acetate and cuprous chloride as catalysts was reported. The chiral high allyl amine compounds can be converted into corresponding β-amino carbonyl compounds with moderate to good yield, good substrate scope, and tolerance of chiral centers. Natural product (+)-sedridine was synthesized with 32% yield.
Key words: β-amino carbonyl compound, chiral high allyl amine, Wacker reaction, (+)-sedridine
PDF全文下载地址:
点我下载PDF
