

1 河北农业大学 动物科技学院,河北 保定 071001;
2 河北科技师范学院 动物科技学院,河北 秦皇岛 066004
收稿日期:2018-02-02;接收日期:2018-05-30 基金项目:河北省现代农业产业技术体系蛋鸡产业创新团队项目(No. HBCT2013090206),河北省科技计划项目(No. 15226302D)资助
摘要:为探明坝上长尾鸡的前黑素小体蛋白(Pre-melanosomal protein,Pmel)基因核心启动子区,首先构建了双荧光素酶表达载体,通过脂质体瞬时转染鸡胚成纤维细胞DF1,并利用双荧光素酶检测试剂盒进行启动活性检测。成功克隆了坝上长尾鸡pmel基因5′侧翼区片段1 268 bp,预测启动子区(-1 200–+68)含有2个CpG岛和多种转录因子结合位点,构建了9个含有不同长度pmel基因启动子片段的表达载体及1个核心启动子区突变载体,说明鸡pmel基因启动子的核心区域为-840–+68 bp,其中-840–-590 bp和-525–-266 bp区域为正调控区,-590–-525 bp区域为负调控区,多态位点(-456、-435、-410、-374和-341)对pmel基因启动子活性有较大影响。
关键词:坝上长尾鸡 pmel基因 启动子 转录因子
Identification of the core promoter of the pmel gene of Bashang long-tail chickens
Xiaohui Liu1, Rongyan Zhou1, Yongdong Peng2, Chuansheng Zhang2, Lanhui Li1, Xianglong Li1,2


1 College of Animal Science and Technology, Hebei Agricultural University, Baoding 071001, Hebei, China;
2 College of Animal Science and Technology, Hebei Normal University of Science & Technology, Qinhuangdao 066004, Hebei, China
Received: February 2, 2018; Accepted: May 30, 2018
Supported by: The Earmarked Fund for Hebei Layer Innovation Team of Modern Agro-industry Technology Research System (No. HBCT2013090206), the Science and Technology Plan Projects of Hebei Province, China (No. 15226302D)
Corresponding author:Xianglong Li. Tel: +86-335-8069521; E-mail: lixianglongcn@yahoo.com
Abstract: To explore the activity of the pmel core promoter of Bashang long-tail chickens, we constructed dual-luciferase expression vectors and transiently transfected into DF1 cells with Lipofectamine 2000. We measured the luciferase activity with the dual-luciferase detection kit. The 1 268 bp fragment in 5′-flanking region of the pmel gene in Bashang long-tail chickens was cloned. The region from -1 200 bp to +68 bp included 2 CpG islands and multiple transcription factor binding sites. We constructed 9 expression vectors with different promoter regions and a mutant vector of the core promoter region of the pmel gene of Bashang long-tail chickens. The core promoter region from –840 bp to +68 bp was identified in the pmel gene. The region from -590 to -525 bp negatively regulated the pmel gene during the transcription process. The -840–-590 bp and -525–-266 bp regions were positive regulatory regions. The polymorphic sites (-456, -435, -410, -374 and -341) had a significant effect on the promoter activity of the pmel gene.
Keywords: Bashang long-tail chicken pmel gene promoter transcription factor
禽羽毛是反映品种和性别的重要表型器官之一,羽毛色泽、形态等外观性状是定价的重要参考指标,羽色的多样性也是自身的天然保护色,坝上长尾鸡就含有多种羽色,包括白、黑、麻、芦花、银、山水等。毛色的形成是一个复杂的过程,不同的表型由一系列毛色基因控制,前人已发现并分析了一些与毛色相关的变异位点,目前小鼠Mus musculus中已经发现378个与毛色相关的基因位点[1]。每一个毛色相关基因都会单独或与其他基因一起对一到多个色素性状起作用[2]。而黑色表型的形成离不开黑色素的沉积,黑色素是由黑色素细胞生成的,黑素体是色素细胞中的膜结合细胞器,合成并储存色素,黑素体是研究细胞膜活动分子机制的完美模型[3-5]。黑色素的形成和沉积主要发生在黑素小体的淀粉样纤维上,前黑素小体蛋白与黑色素的产生和沉积关系紧密,在黑色素的合成和贮存中发挥重要作用[6]。已有研究报道,在高原牛Bos taurus中,peml突变使得真黑素和褐黑素均受到影响[7]。pmel基因的突变会引起马Equus caballus的银色表型[8-9],鸡Gallus gallus羽色呈烟灰色或白色[10]。小鼠pmel失活时黑素体会变成球形,而野生型小鼠黑素体是椭圆形,pmel失活引起毛发中真黑素大量减少,在维持表皮色素沉积上有重要作用,但对小鼠毛色表型仅有轻微影响[11]。鸡peml基因位于33号染色体(GenBank登录号:NC_008465.3),含有11个外显子,长约4 kb,编码740个氨基酸(数据库:https://www.ncbi.nlm.nih.gov/)。
启动子区决定转录起始,调控基因表达的强弱,研究基因启动子转录调控具有非常重要的作用。关于山羊Capra hircas和水貂Mustla vison的peml启动子已有研究,并且证明Sp1是其重要的正调控元件[12-13]。本研究通过鸡peml基因启动子活性分析,以明确该基因启动子调控区,预测启动子区结构及候选转录因子结合位点,为研究鸡peml基因的转录调控提供理论基础。
1 材料与方法1.1 试验样品试验用坝上长尾鸡的血液样品来源于张家口京星园生态农业有限公司,采集60周龄坝上长尾鸡的黑羽母鸡翅根部静脉血,共采集53个样本。
1.2 试验试剂基因组提取试剂盒、Taq酶、PCR纯化试剂盒、DNA分子量标准、Trans5α感受态、染色剂、胎牛血清购自北京全式金公司。pMD19-T (Simple)、DNA连接试剂盒、KpnⅠ和HindⅢ购自宝生物工程(大连)有限公司。DNA小量抽提试剂盒、胶回收试剂盒购自生工生物工程(上海)股份有限公司。无内毒素质粒大提试剂盒购自天根生化科技有限公司。双荧光素酶报告基因检测试剂盒购自Promega公司。DMEM、DPBS (Hyclone,美国),T4 DNA连接酶、0.25%胰蛋白酶、Opti-MEM培养基和Lipofectamine?2000 (Thermo Fisher Science,美国),pGL3-Basic质粒、pRL-TK质粒(平皓生物技术有限公司,中国北京),鸡成纤维细胞(DF1) (北京协和细胞资源库,中国北京)。
1.3 方法1.3.1 启动子序列分析通过UCSC数据库(http://genome.ucsc.edu/)、NCBI数据库(https://www.ncbi.nlm.nih.gov/)、BioEdit7.0.9分析序列,用Primer premier 5.0设计引物分析限制性酶切位点,通过AliBaba2.1数据库(http://gene-regulation.com/pub/programs/alibaba2/ index.html)预测转录因子结合位点,用MethPrimer软件(http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi)预测CpG岛。
1.3.2 PCR扩增和载体构建从NCBI数据库获取鸡pmel基因上游序列(GenBank登录号:NC_008465.3) 2 000 bp,利用Primer premier 5.0设计引物,分析限制性内切酶位点,引入KpnⅠ和HindⅢ,并加上保护碱基,扩增出不同长度的缺失片段(表 1)。PCR扩增体系:Taq酶0.5 μL,缓冲液5 μL,2.5 mmol/L dNTPs 4 μL,DNA (50 ng/μL) 5 μL,上下游引物(10 μmol/L)各1 μL,ddH2O补足至50 μL。PCR反应条件:95 ℃ 5 min;95 ℃ 30 s,退火30 s,72 ℃延伸,共35个循环(退火温度和延伸时间见表 1);72 ℃延伸10 min,4 ℃保存。PCR产物通过1%琼脂糖凝胶电泳检测。
表 1 实验所用引物Table 1 Primers used in this study
| Name | Primer sequence (5′–3′) | Size (bp) | Tm (℃) | Extension time (s) |
| p1-F | GGGGTACCTGGGGGAGGAACGGGTTG | 1 269 | 60 | 80 |
| p2-F | GGGGTACCCGCCGTCCCATTGGCTGAG | 909 | 62 | 60 |
| p3-F | GGGGTACCAGATCCCTTAAAGCGACAGGC | 862 | 60 | 55 |
| p4-F | GGGGTACCTTGTTATCAGGCTGCCCAC | 708 | 61 | 45 |
| p5-F | GGGGTACCTTCCCAGGCAAACGGTCC | 659 | 60 | 38 |
| p6-F | GGGGTACCGTCATACACGCAGACCCAC | 614 | 58 | 35 |
| p7-F | GGGGTACCCACAGGGTCCCCACAGCC | 594 | 62 | 35 |
| p8-F | GGGGTACCTCCTGCCAGCCACAGCCAC | 472 | 64 | 30 |
| p9-F | GGGGTACCTCCAGGGTCACATCCATAACAC | 335 | 60 | 20 |
| p1—p9-R | CCCAAGCTTCGTGCAACCGCATGCTTC | |||
| Note: Bold types (GG and CC) are protective bases, and underlined sequences (GGTACC and CAAGCTT) are restriction enzyme cutting sites, F represents forward primer, R represents reverse primer. | ||||
表选项
PCR产物经琼脂糖凝胶回收纯化,与pMD19-T (Simple)重组,分别对重组T载体和pGL3-Basic双酶切,回收目的片段,用T4 DNA连接酶连接,将重组的表达载体P1转化至Trans5α感受态细胞。再以P1为模板依次构建P2–P9,对酶切和测序鉴定为阳性的菌液提取无内毒素质粒并检测其浓度和纯度。
1.3.3 启动子区多态检测和突变载体的构建从UCSC数据库公布的数据可以看出pmel启动子区存在单核苷酸多态(Single nucleotide polymorphisms,SNPs) (图 1),以53只坝上长尾鸡DNA为模板,利用核心启动子区的引物,进行PCR扩增,并送至北京六合华大基因有限公司进行测序,分析序列多态位点。
 |
| 图 1 坝上长尾鸡pmel基因上游序列与UCSC数据库比对结果 Figure 1 Alignment of upstream sequence of the Bashang long-tail chicken pmel gene with the UCSC database. |
| 图选项 |
选择核心启动子区要获得的多态位点与已获得的核心启动子表达载体该位点碱基不同或为杂合的PCR产物,按照以上载体构建的方法,构建核心启动子区突变载体并进行测序,挑选符合要求的载体,提取无内毒素质粒并检测其浓度和纯度。
1.3.4 细胞培养和活性检测DF1细胞培养在37 ℃、5% CO2条件下进行,DMEM含10%胎牛血清,细胞状态良好时进行细胞计数铺于24孔板内,24 h细胞贴壁覆盖底部80%左右时进行转染,每孔表达载体(0.475 μg)、pRL-TK (0.025 μg)、脂质体(1.0 μL)和Opti-MEM共50 μL混匀孵育5 min,转染到DF1细胞,48 h检测萤火虫与海肾荧光素酶的相对活性。实验进行3次系统重复,每次包含3次技术重复。
1.3.5 统计分析将测得萤火虫荧光素酶活性检测值F除以海肾荧光素酶活性检测值R,得到相对值F/R,再除以对照pGL3-Basic的相对荧光素酶活性检测的背景值,以消除不同转染批次的系统误差。利用SPSS19.0软件对试验数据进行Kruskal-Wallis H(K)非参数检验。
2 结果与分析2.1 坝上长尾鸡pmel基因启动子扩增与序列分析以坝上长尾鸡基因组为模板,进行PCR扩增获得了鸡pmel基因候选启动子区域1 268 bp,并对扩增产物进行了测序,与UCSC提交的pmel基因序列(Chromosome 33: 616 856–618 124)相似性为99.2% (图 1),说明片段来源正确,可用于试验,将此处外显子1起始位点作为+1。发现该基因的-1 152–-483 bp和-150–+12 bp处分别存在670 bp和162 bp的CpG岛(图 2)。启动子区(-840–-266)预测到转录因子结合位点:Sp1、AP-2、YY1、GATA-1、NF-κB、USF、SRF、Oct-1、C/EBP、CREB、RAP1转录因子结合位点。Sp1在-840–-793 bp和-403–-266 bp区域分布最为集中,-793–-590 bp区域较为集中,-590–-403 bp区域最少。
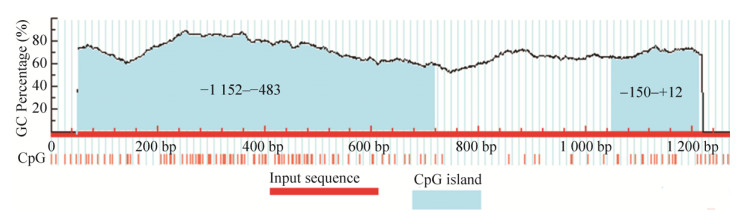 |
| 图 2 坝上长尾鸡pmel基因从–1 200到+68 bp序列中CpG岛预测 Figure 2 CpG islands in the pmel gene from –1 200 to +68 bp predicted by the MethPrimer software. |
| 图选项 |
2.2 坝上长尾鸡pmel启动子区不同缺失片段的扩增利用不同引物对鸡pmel基因候选启动子区域进行缺失片段的扩增,包括p1 (-1 200–+68)、p2 (-840–+68)、p3 (-793–+68)、p4 (-639–+68)、p5 (-590–+68)、p6 (-545–+68)、p7 (-525–+68)、p8 (-403–+68)、p9 (-266–+68),1%琼脂糖凝胶电泳检测,缺失片段长度与预期一致。
2.3 坝上长尾鸡pmel启动子荧光素酶表达载体的构建与活性分析扩增片段p1与pMD-19T载体连接体系:1 μL pMD-19T载体,1–4 μL PCR回收产物,5 μL SolutionⅠ,用ddH2O补足至10 μL,16 ℃连接,对重组T载体进行双酶切,将目的片段通过T4 DNA连接酶连接到表达载体pGL3-Basic,构建表达载体P1,再以P1为模板依次构建P2–P9,对表达载体(P1–P9及Basic)进行双酶切(图 3)、测序鉴定,提取无内毒质粒并检测其浓度和纯度。
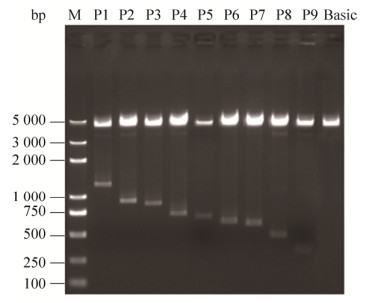 |
| 图 3 表达载体双酶切产物凝胶电泳 Figure 3 Agarose gel electrophoresis of expression vectors by dual-enzyme digestion. M: Trans2K? Plus DNA Marker; P1–P9: Expression vectors with different lengths of promoter regions, corresponding to –1 200– +68, –840–+68, –793–+68, –639–+68, –590–+68, –545–+68, –525–+68, –403–+68 and –266–+68, respectively; Basic: blank vector; The same as Fig. 4. |
| 图选项 |
以不同的表达载体转染DF1细胞,进行双荧光素酶活性检测,图 4可知表达载体P1–P9的活性与Basic有显著差异(P < 0.05),报告基因P2 (-840–+68)转染活性值最高,该活性值是对照组的40倍,表明-840–+68 bp区域为鸡pmel基因的核心启动子区域,-840–-590 bp和-525–-266 bp区域是重要的正调控区,-590–-525 bp区域是重要的负调控区。
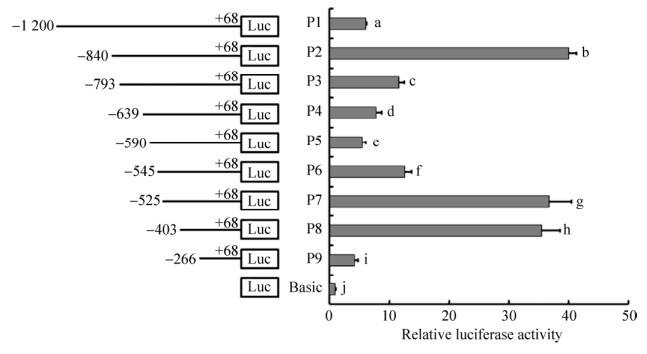 |
| 图 4 启动子转染DF1细胞的相对荧光活性值 Figure 4 Relative activity of the promoters in DF1 cells. On the right side, the standard errors were represented by the error lines. Different lowercase letters indicate significant difference (P < 0.05). The same as Fig. 6. |
| 图选项 |
2.4 坝上长尾鸡pmel启动子区基因型检测坝上长尾鸡pmel启动子-840–-185 bp区域检测到12个多态位点,其中-732、-654、-410、-326、-185位点在UCSC数据库中未公布(表 2),启动子活性降低最为显著的两个区域是-840–-793 bp和-403–-266 bp,且-525–-266 bp区域是重要的正调控区,-840–-793 bp区域未检测到多态,-525–-266 bp区域检测到7个多态位点。
表 2 坝上长尾鸡多态位点的等位基因和基因型频率Table 2 The allele and genotype frequency of the polymorphic sites of Bashang long-tail chickens.
| Position (bp) | UCSC reference SNP ID | Genotype frequency | Allele frequency | |||
| –732 | No ID | GG (0.667) | GC (0.167) | CC (0.167) | G (0.750) | C (0.250) |
| –654 | No ID | TT (0.250) | TG (0.500) | GG (0.250) | T (0.500) | G (0.500) |
| –549 | rs315175669 | TT (0.333) | TC (0.500) | CC (0.167) | T (0.583) | C (0.417) |
| –456 | rs317535292 | TT (0.667) | TC (0.250) | CC (0.083) | T (0.792) | C (0.208) |
| –435 | rs14684285 | TT (0.250) | TC (0.333) | CC (0.417) | T (0.417) | C (0.583) |
| –410 | No ID | TT (0.667) | TG (0.333) | GG (0.000) | T (0.833) | G (0.167) |
| –374 | rs14684284 | AA (0.250) | AG (0.250) | GG (0.500) | A (0.375) | G (0.625) |
| –341 | rs14684283 | CC (0.000) | CG (0.333) | GG (0.667) | C (0.167) | G (0.833) |
| –326 | No ID | GG (0.750) | GA (0.250) | AA (0.000) | G (0.875) | A (0.125) |
| –270 | rs314277925 | GG (0.750) | GA (0.000) | AA (0.250) | G (0.750) | A (0.250) |
| –204 | rs14684282 | TT (0.250) | TC (0.417) | CC (0.333) | T (0.458) | C (0.542) |
| –185 | No ID | TT (0.667) | TC (0.250) | CC (0.083) | T (0.792) | C (0.208) |
表选项
2.5 坝上长尾鸡pmel核心启动子区突变载体的构建与活性分析选择核心启动子区内-525–-266 bp区域7个多态位点为杂合或与P2这7个位点碱基不同,其他多态位点为纯合的序列,按照以上载体构建的方法,构建核心启动子区突变载体(mut-1) (图 5),转染DF1细胞,对报告基因进行双荧光素酶活性检测,mut-1显著高于P2的活性值(P < 0.05) (图 6),由此推测,-456、-435、-410、-374、-341位点多态的发生对pmel启动子活性有影响。
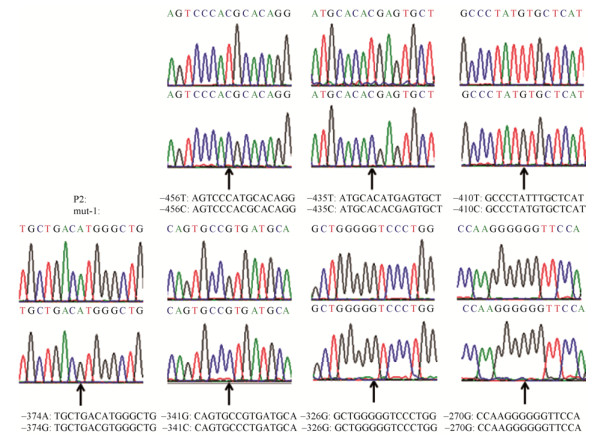 |
| 图 5 P2和mut-1多态位点所在区域测序结果(箭头提示多态位点) Figure 5 Sequencing results of the polymorphic sites in P2 and mut-1. The arrows indicated the polymorphic sites. The sequence of mut-1 is consistent with P2 except –456, –435, –410, –374 and –341 sites. |
| 图选项 |
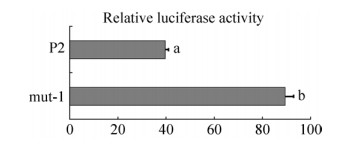 |
| 图 6 核心启动子区的活性分析 Figure 6 Activity analysis of the core promoters. The sequence of mutant vector (mut-1) is consistent with luciferase expression vector (P2) except –456, –435, –410, –374 and –341 sites. |
| 图选项 |
3 讨论转录调控是基因表达过程中重要的一步,转录起始是众多顺式作用元件和反式作用因子之间相互作用的结果。pmel基因是影响羽色形成的重要基因,通过和其他基因互作对皮肤毛色形成以及其他组织色素形成均有作用,本研究构建了9个含有不同长度鸡pmel基因启动子片段的表达载体(P1–P9)及1个核心启动子区突变载体(mut-1),确定了鸡pmel基因启动子的核心区域为-840–+68 bp,-840–-590 bp和-525–-266 bp区域为正调控区,存在多个转录因子(Sp1、AP-2、GATA-1、NF-κB、YY1、USF、SRF、Oct-1和C/EBP)结合位点,推测这些转录因子可能对pmel启动子起正调控作用。
启动子从–840 bp截短至–793 bp、–403 bp截短至–266 bp启动子活性降低最为明显,启动子相对活性值P2是P3的3.44倍,P8是P9的8.4倍,该区域也是Sp1分布最为集中的区域,从–793 bp截短至–590 bp启动子活性降低较为显著,启动子相对活性P3是P5的2.12倍,Sp1分布较为集中。Sp1的DNA结合区域特异性地识别GC盒(GGGGCGGGG)与GT盒(GGTGTGGG)[14],GC/GT常以多拷贝形式分布于基因的启动子和/或增强子中[15],Sp1也可直接与转录起始复合物中的TFIID复合物相互作用。在低氧状态下人内皮型一氧化氮合酶(eNOS)启动子GC富集区插入Sp1结合位点,启动子活性增强,可减轻部分心血管疾病中存在的微循环障碍[16]。此外Sp1也可与多种转录因子共同调控基因表达,例如,Sp1和AP-2的表达上调或下降共同调控不同靶基因的表达[17]。转录因子YY1在多种组织中广泛表达,YY1能作为启动元件结合蛋白启动转录,通过与其他蛋白的相互作用发挥间接激活或抑制,YY1与Sp1共同作用于HAS2基因的表达[18-19]。USF可与启动子上E-box顺式调控序列结合[20-21],低氧条件下,过表达Sp1和USF激活ADAMTS1启动子活性,增强其转录和表达水平,Sp1和USF异常表达会降低ADAMTS1的转录和蛋白表达量[22]。SRF可与SMYD1启动子上CArG位点结合上调SMYD1在C2C12细胞中的表达[23]。GATA-1是转录因子GATA蛋白家族成员之一,可以通过(A/T) GATA (A/G)与基因启动子结合,激活基因转录[24]。Sp1协同Gata3、MafB、Gcm2转录因子激活甲状旁腺素基因的表达[25-26]。NF-κB是一种重要的细胞内信号因子,由P50和P65组成的二聚体,调节许多与细胞生长和分化相关的基因表达,冯怡的研究表明NF-κB上调SET基因的表达[27]。Toll样受体9 (tlr9)在黑素原生成过程中具有重要作用,与色素增强或减退相关的疾病关系密切,在人黑素细胞系(PIG1)黑素合成过程中NF-κB通过激活tlr9调控黑色素的合成,对人pmel基因的转录和表达有增强作用[28]。CCAAT增强子结合蛋白(C/EBP)家族是碱性亮氨酸拉链蛋白家族的一个亚家族,其蛋白质分子包括3个相似的结构组分:C末端的亮氨酸拉链,N末端的转录激活域,中间的DNA结合域,结合并转录激活特定基因DNA增强子5?-RTTGCGYAAY-3?(R=A或G,Y=C或T)重复序列或其变异体,故可以对基因的转录进行正、负调控[29-31]。已有研究表明,小鼠上过表达Oct-1后使黑素细胞中mitf的表达降低,tyr、tyrp1的表达增加,改变毛色[32]。
启动子-590–-403 bp区域Sp1分布最少,负调控区-590–-525还存在转录因子调控位点CREB、RAP1。已有研究表明,SKOV3细胞增殖受rap1基因下调的影响,卵巢浆液性癌组织中Rap1蛋白表达较高,其会对浆液性癌细胞增殖进行抑制[33]。CREB是真核生物细胞核内调控因子,参与中枢神经系统的可塑性形成以及疾病发生等复杂的生理病理过程,可以刺激基因转录,通过自身的磷酸化实现转录调节的功能,故又称为转录增强因子[34]。而郭姗姗的研究表明CREB对app的表达有下调作用[35]。本实验中P6没有CREB转录因子结合位点,活性显著大于P5,进一步截去Rap1转录因子结合位点时,P7启动子活性显著增强,推测CREB、Rap1可能对pmel启动子起负调控作用。有研究表明人无心肌细胞启动子区多态与内皮型一氧化氮合酶表达减少相关,会引起心室衰竭、血管收缩障碍[36]。本实验pmel启动子-840–-185 bp区域检测到12个多态位点,其中-525–-266 bp区域检测到7个多态位点。表达载体mut-1显著高于P2的活性值(P < 0.05),由此推测-456、-435、-410、-374、-341位点多态的发生对pmel启动子活性有影响,可能是多态的出现改变了转录因子与序列的结合。由于基因的转录调控是受到多种调控元件的协同作用,仍需通过染色质免疫共沉淀和电泳迁移变动分析试验进一步研究其作用机制。
参考文献
| [1] | Montoliu L, Oetting WS, Bennett DC. Color Genes. (10, 2011). European society for pigment cell research[EB/OL].[2011-10-02]. http://www.espcr.org/micemut. |
| [2] | Hubbard JK, Uy JAC, Hauber ME, et al. Vertebrate pigmentation: from underlying genes to adaptive function.Trends Genet, 2010, 26(5): 231–239.DOI: 10.1016/j.tig.2010.02.002 |
| [3] | Hearing VJ. The melanosome: the perfect model for cellular responses to the environment.Pigment Cell Res, 2000, 13(S8): 23–34. |
| [4] | Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white.Nat Rev Mol Cell Biol, 2001, 2(10): 738–748.DOI: 10.1038/35096009 |
| [5] | Seiji M, Fitzpatrick TB, Birbeck MSC. The melanosome: a distinctive subcellular particle of mammalian melanocytes and the site of melanogenesis.J Invest Dermatol, 1961, 36(4): 243–252. |
| [6] | Du ZY, Xu C, Song XC, et al. Regulating mechanism of Peml17 in the maturation process of melanosomes.J Domest Anim Ecol, 2016, 37(2): 1–7.(in Chinese). 杜站宇, 徐超, 宋兴超, 等. Pmel17在黑素小体成熟过程中的关键作用.家畜生态学报, 2016, 37(2): 1-7. |
| [7] | Schmutz SM, Dreger DL. Interaction of MC1R and PMEL alleles on solid coat colors in Highland cattle.Anim Genet, 2013, 44(1): 9–13.DOI: 10.1111/age.2013.44.issue-1 |
| [8] | Reissmann M, Bierwolf J, Brockmann GA. Two SNPs in the SILV gene are associated with silver coat colour in ponies.Anim Genet, 2007, 38(1): 1–6.DOI: 10.1111/age.2007.38.issue-1 |
| [9] | Brunberg E, Andersson L, Cothran G, et al. A missense mutation in PMEL17 is associated with the Silver coat color in the horse.BMC Genet, 2006, 7: 46. |
| [10] | Kerje S, Sharma P, Gunnarsson U, et al. The Dominant white, Dun and Smoky color variants in chicken are associated with insertion/deletion polymorphisms in the PMEL17 gene.Genetics, 2004, 168(3): 1507–1518.DOI: 10.1534/genetics.104.027995 |
| [11] | Hellstr?m AR, Watt B, Fard SS, et al. Inactivation of Pmel alters melanosome shape but has only a subtle effect on visible pigmentation.PLoS Genet, 2011, 7(9): e1002285.DOI: 10.1371/journal.pgen.1002285 |
| [12] | Li LS, Li LH, Li XL, et al. Analysis of the promoter activity and transcriptional regulatory elements of PMEL gene in mink (Mustela vison).J Agric Biotechnol, 2017, 25(6): 911–920.(in Chinese). 李丽莎, 李兰会, 李祥龙, 等. 水貂PMEL基因启动子活性及转录调控元件分析.农业生物技术学报, 2017, 25(6): 911-920. |
| [13] | Li LS, Peng YD, Zheng XN, et al. Analysis of the promoter activity and transcriptional regulatory elements of goat PMEL gene.Chin J Anim Veter Sci, 2017, 48(5): 826–835.(in Chinese). 李丽莎, 彭永东, 郑晓宁, 等. 山羊PMEL基因启动子活性及转录调控元件分析.畜牧兽医学报, 2017, 48(5): 826-835. |
| [14] | Yu B, Datta PK, Bagchi S. Stability of the Sp3-DNA complex is promoter-specific: Sp3 efficiently competes with Sp1 for binding to promoters containing multiple Sp-sites.Nucleic Acids Res, 2003, 31(18): 5368–5376.DOI: 10.1093/nar/gkg706 |
| [15] | Emili A, Greenblatt J, Ingles CJ. Species-specific interaction of the glutamine-rich activation domains of Sp1 with the TATA box-binding protein.Mol Cell Biol, 1994, 14(3): 1582–1593.DOI: 10.1128/MCB.14.3.1582 |
| [16] | Yang YH, Liu S, Fan ZH, et al. Sp1 modification of human endothelial nitric oxide synthase promoter increases the hypoxia-stimulated activity.Microvasc Res, 2014, 93: 80–86.DOI: 10.1016/j.mvr.2014.03.004 |
| [17] | Yong XU, Porntadavity S, St Clair DK. Transcriptional regulation of the human manganese superoxide dismutase gene: the role of specificity protein 1 (Sp1) and activating protein-2 (AP-2).Biochem J, 2002, 362(2): 401–412.DOI: 10.1042/bj3620401 |
| [18] | Jokela TA, Makkonen KM, Oikari S, et al. Cellular Content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1.J Biol Chem, 2011, 286(38): 33632–33640.DOI: 10.1074/jbc.M111.265637 |
| [19] | Smale ST, David B. The "initiator" as a transcription control element.Cell, 1989, 57(1): 103–113.DOI: 10.1016/0092-8674(89)90176-1 |
| [20] | Li SF, Hua ZC. Expression, purification of human transcription factor USF and its DNA binding analysis in FAK promoter.J Southeast Univ: Med Sci Ed, 2007, 26(4): 267–270.(in Chinese). 李淑锋, 华子春. 人转录因子USF基因在大肠杆菌中的表达、纯化及在FAK启动子中的结合研究.东南大学学报:医学版, 2007, 26(4): 267-270. |
| [21] | Tan YW. Transcriptional down-regulation of smurf1/2 ubiquitin ligase by upstream stimulate factor 2 (USF2)[D]. Ji'nan: Shandong University, 2013 (in Chinese). 谭雅文.上游刺激因子2(USF2)对Smurf1和Smurf2的功能调控研究[D].济南: 山东大学, 2013. |
| [22] | Turkoglu SA, Kockar F. SP1 and USF differentially regulate ADAMTS1 gene expression under normoxic and hypoxic conditions in hepatoma cells.Gene, 2016, 575(1): 48–57.DOI: 10.1016/j.gene.2015.08.035 |
| [23] | Wang J, Ye XL, Jiang L, et al. IGF-1 regulates SMYD1 expression through SRF response element in C2C12 cells.Chin J Biochem Mol Biol, 2010, 26(12): 1113–1120.(in Chinese). 王娟, 叶湘漓, 姜丽, 等. IGF-1通过SRF结合位点调节SMYD1在C2C12细胞中的表达.中国生物化学与分子生物学报, 2010, 26(12): 1113-1120. |
| [24] | Tian KG, Fu M, Gao Y, et al. Binding of transcription factor GATA-1 to 5′ upstream sequence of human cytomegalovirus UL111A gene.Chin J Pathophysiol, 2012, 28(12): 2244–2249.(in Chinese). 田可港, 浮苗, 高艳, 等. 转录因子GATA-1与人巨细胞病毒UL111A基因5'上游序列结合的实验研究.中国病理生理杂志, 2012, 28(12): 2244-2249.DOI:10.3969/j.issn.1000-4718.2012.12.024 |
| [25] | Han SI, Tsunekage Y, Kataoka K. Gata3 cooperates with Gcm2 and MafB to activate parathyroid hormone gene expression by interacting with SP1.Mol Cell Endocrinol, 2015, 411: 113–120.DOI: 10.1016/j.mce.2015.04.018 |
| [26] | Schanton M, Maymó J, Pérez-Pérez A, et al. Sp1 transcription factor is a modulator of estradiol leptin induction in placental cells.Placenta, 2017, 57: 152–162.DOI: 10.1016/j.placenta.2017.07.005 |
| [27] | Feng Y. Part one human set gene expression by NF-κB signaling activation[D]. Chongqing: Chongqing Medical University, 2016 (in Chinese). 冯怡. NF-κB上调SET基因的表达及其分子机制研究[D].重庆: 重庆医科大学, 2016. |
| [28] | Sun LJ, Pan SJ, Yang YJ, et al. Toll-like receptor 9 regulates melanogenesis through NF-κB activation.Exp Biol Med, 2016, 241(14): 1497–1504.DOI: 10.1177/1535370216642529 |
| [29] | Landschulz WH, Johnson PF, Mcknight SL. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite.Science, 1989, 243(4899): 1681–1688.DOI: 10.1126/science.2494700 |
| [30] | Roman C, Platero JS, Shuman J, et al. Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is similar to C/EBP and heterodimerizes with C/EBP.Gene Dev, 1990, 4(8): 1404–1415.DOI: 10.1101/gad.4.8.1404 |
| [31] | Williams SC, Cantwell CA, Johnson PF. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro.Gene Dev, 1991, 5(9): 1553–1567.DOI: 10.1101/gad.5.9.1553 |
| [32] | Yang YJ, Nie RQ, Xie JS, et al. The influences of over-expression of Oct-1 on major genes of coat color in melanocytes of mice.Sci Agric Sin, 2017, 50(4): 764–773.(in Chinese). 杨玉静, 聂瑞强, 谢建山, 等. 小鼠黑素细胞中过表达Oct-1对毛色主效基因的影响.中国农业科学, 2017, 50(4): 764-773. |
| [33] | Hou XW, Chen LX. Investigating the expression of Rap1 protein in ovarian serous carcinoma and its role in the proliferation of serous cancer cells.Chin Foreign Med Res, 2017, 15(25): 55–56.(in Chinese). 侯祥位, 陈立侠. 探讨卵巢浆液性癌组织中Rap1蛋白表达及其在浆液性癌细胞增殖中的作用.中外医学研究, 2017, 15(25): 55-56. |
| [34] | Qiao FZ, Zhang YB, Niu JZ. The role of CERB on pathologic pain and pain modulation.J Taishan Med Coll, 2017, 38(10): 1198–1200.(in Chinese). 乔福珍, 张颜波, 牛敬忠. CERB在病理性痛痛觉调制中的作用研究.泰山医学院学报, 2017, 38(10): 1198-1200.DOI:10.3969/j.issn.1004-7115.2017.10.046 |
| [35] | Guo SS, Zhang BY, He WX, et al. The establishment of stable CREB gene knock out cell lines with CRISPR/Cas9 technique and the regulatory effects of CREB on APP gene expression.Chin J Cell Biol, 2017, 39(9): 1147–1155.(in Chinese). 郭姗姗, 张冰莹, 何文欣, 等. 利用CRISPR/Cas9技术构建CREB基因敲除细胞系并探讨CREB对APP基因表达的调控作用.中国细胞生物学学报, 2017, 39(9): 1147-1155. |
| [36] | Doshi AA, Ziolo MT, Wang HL, et al. A Promoter polymorphism of the endothelial nitric oxide synthase gene is associated with reduced mRNA and protein expression in failing human myocardium.J Card Fail, 2010, 16(4): 314–319.DOI: 10.1016/j.cardfail.2009.12.013 |
