檀利军1, 王敬敬1,2,3, 石千黛1, 刘海泉1,2,3, 赵勇1,2,3


1. 上海海洋大学食品学院, 上海 201306;
2. 上海水产品加工贮藏工程技术研究中心, 上海 201306;
3. 农业农村部水产品贮藏保鲜质量安全风险评估实验室(上海), 上海 201306
收稿日期:2019-11-28;修回日期:2020-04-03;网络出版日期:2020-06-14
基金项目:国家自然科学基金(31671779,31972188);国家重点研发计划(2018YFC1602205);上海市科技兴农项目(沪农科推字2017第4-4号);上海市教育委员会科研创新计划(2017-01-07-00-10-E00056)
*通信作者:赵勇, Tel:+86-21-61900503;E-mail:yzhao@shou.edu.cn.
摘要:副溶血性弧菌(Vibrio parahaemolyticus)是海产品中一种常见的食源性致病菌,常导致水产养殖动物患病或者引起食物中毒。耐热性直接溶血素(thermotolerant direct hemolysin,TDH)是副溶血性弧菌最为重要的致病因子之一。本文围绕tdh基因在弧菌属中的广泛分布与传播、tdh基因的多样性及其表达调控、TDH的蛋白结构及其生物活性进行了综述,并对未来TDH的研究方向进行了展望。旨在进一步了解由副溶血性弧菌感染所引起的病症,为预防副溶血性弧菌的感染和临床治疗提供理论支撑。
关键词:副溶血性弧菌耐热性直接溶血素tdh基因结构表达调控生物活性
Advances in research on thermostable direct hemolysin (TDH) of Vibrio parahaemolyticus
Lijun Tan1, Jingjing Wang1,2,3, Qiandai Shi1, Haiquan Liu1,2,3, Yong Zhao1,2,3


1. College of Food Science and Technology, Shanghai Ocean University, Shanghai 201306, China;
2. Shanghai Engineering Research Center of Aquatic-Product Processing & Preservation, Shanghai 201306, China;
3. Quality & Safety Risk Assessment for Aquatic Products on Storage and Preservation(Shanghai), Ministry of Agriculture, Shanghai 201306, China
Received: 28 November 2019; Revised: 3 April 2020; Published online: 14 June 2020
*Corresponding author: Yong Zhao, Tel: +86-21-61900503; E-mail:yzhao@shou.edu.cn.
Foundation item: Supported by the National Natural Science Foundation of China (31671779, 31972188), by the National Key R & D Program ofChina (2018YFC1602205), by the Shanghai Agriculture Applied Technology Development Program (T20170404) and by theInnovation Program of Shanghai Municipal Education Commission (2017-01-07-00-10-E00056)
Abstract: Vibrio parahaemolyticus is a widely distributed foodborne pathogen in seafood, often leading to diseases in aquaculture animals or food poisoning for human beings. Thermotolerant direct hemolysin (TDH) is one of the most important virulence factors of V. parahaemolyticus. This article systematically reviews the widespread distribution, spreading, the diversity and expression regulation of tdh gene, as well as the structure, biological activity and promising research directions of TDH protein. The article aims to deeply understand the symptoms caused by V. parahaemolyticus infection, and hence to provide theoretical support for the prevention and clinical treatment of V. parahaemolyticus infection.
Keywords: Vibrio parahaemolyticusthermotolerant direct hemolysintdh genestructureexpression regulationbiological activity
副溶血性弧菌是一种常见的革兰氏阴性嗜盐型海洋细菌,主要分布在鱼类、虾类、贝类等海产品中。副溶血性弧菌可导致水产养殖动物疾病,给水产养殖业带来巨大的经济损失。更严重的是,人类如果食用了由副溶血性弧菌污染而未煮熟的海产品容易引起海鲜性胃肠炎、炎症性腹泻等[1-3]。此外,副溶血性弧菌还与伤口感染和败血症有关[4]。近年来,由副溶血性弧菌引起的食物中毒已经成为世界范围内的公共卫生问题[2]。因此,对副溶血性弧菌的研究日益引起社会关注,其毒力因子和致病机理的研究也受到越来越多的重视。
副溶血性弧菌的致病过程包括侵袭、粘附、体内增殖及产生毒素等过程[5]。这些过程与副溶血性弧菌产生的多种毒力因子息息相关,主要包括溶血毒素、Ⅲ型/Ⅵ型分泌系统、粘附因子、铁摄取系统、脂多糖、蛋白酶和外膜蛋白等[5]。溶血毒素是副溶血性弧菌最为重要的毒力因子之一,它是一种能够溶解哺乳动物的红细胞膜并释放血红蛋白的外毒素,分为耐热性直接溶血素(TDH),耐热性相关溶血素(thermotolerance related hemolysin,TRH)和不耐热性溶血素(thermolabile hemolysin,TLH),其中TDH由tdh基因编码[4]。本文综述了TDH近年来的一些研究进展,旨在进一步了解由副溶血性弧菌感染引起的疾病,为预防副溶血性弧菌的感染和临床治疗提供理论支撑。
1 tdh基因在弧菌属中的广泛分布与传播 除了在副溶血性弧菌中存在tdh基因外,在其他弧菌属中也发现了tdh基因[6-8]。用tdh基因探针检测发现,所有的霍利斯弧菌(V. hollisae)均含有tdh基因(命名为Vh-tdh);从临床上分离的部分非O1型霍乱弧菌(V. cholerae non-O1)和拟态弧菌(Vibrio mimicus)也携带tdh基因(分别命名为NAG-tdh和Vm-tdh)。通过克隆tdh基因并测序后发现,这3种弧菌的tdh基因和副溶血性弧菌的tdh基因有着非常高的同源性,与tdh2 (副溶血性弧菌tdh基因中的一种)的核苷酸序列相似度分别为93.3%、98.6%、97.0%[9] (表 1)。此外,非O1型霍乱弧菌所携带的NAG-tdh和副溶血性弧菌AQ3776所携带的tdh4 (副溶血性弧菌tdh基因中的一种)都位于质粒上,两者的tdh基因核苷酸序列相似度为100%[6],并且在这两种菌株中,携带tdh基因的质粒整体上也有高度同源性。这些结果证明,质粒可介导tdh基因在副溶血性弧菌和霍乱弧菌之间进行转移传播[6]。但是,临床分离的副溶血性弧菌基本上都携带tdh基因,而临床分离的非O1型霍乱弧菌却极少携带tdh基因,所以更倾向于推测tdh基因最初是通过副溶血性弧菌由质粒作为载体转移到非O1型霍乱弧菌当中的。一般情况下,副溶血性弧菌tdh基因位于其2号染色体上的致病岛区域[10] (图 1),致病岛的获取与水平基因转移或噬菌体有关[11]。因此tdh基因可能通过质粒与染色体在弧菌属之间进行转移传播。在各弧菌属中,tdh基因的侧翼是类插入序列(insertion sequence in vibrios,ISVs)或源自ISVs基因重排的相关序列[12]。ISVs的核苷酸序列与插入序列(insertion sequence,IS)家族中的IS903相似,具有与IS903高度同源的18 bp末端反向重复序列。IS903编码的转座酶具有转座活性,但ISVs编码的转座酶却没有活性。因此,ISVs无法促使tdh基因在弧菌属中进行转移,tdh基因在副溶血性弧菌的染色体上是稳定存在的[12]。有人据此提出如下关于副溶血性弧菌tdh基因起源的假说[9, 12]:ISVs序列的G+C含量(43.7%–46.6%)接近弧菌属基因组的平均G+C含量(46.0%–49.0%),但tdh基因的G+C含量(31.5%–33.7%)却明显比前两者要低。ISVs在过去的某个时期可能具有转座活性,通过转座将tdh基因在不同菌种之间进行转移,质粒可能是不同菌种之间tdh基因转移的载体。通过这种机制,tdh基因首先从带有tdh基因的非弧菌菌株转移到弧菌中,然后在不同弧菌之间进行转移。经过了长期的进化,ISVs序列发生了很大的变异,失去了转座活性。另外一个可能的机制是转导,但目前尚没有证据表明噬菌体参与了tdh基因的转移。大多数ISV转座酶基因序列是由于碱基的改变和缺失而发生了改变[12],在拟态弧菌中发现的一个ISV-M5.2则包含可能编码活性转座酶的开放阅读框(open reading frame,ORF),但未能检测到该转座酶的活性。这些结果表明,引入到弧菌中的tdh基因可能已经在其染色体上固定,而tdh基因两侧对活性的ISVs缺乏选择性压力,这使得这些ISVs容易多样化和缺失,同时tdh基因编码序列变得不易转移与传播[12]。
表 1. 确定核苷酸序列的tdh基因[9] Table 1. tdh genes for which the nucleotide sequences were determined[9]
| Desination | Species | Strain | Source | KPa | Location | Similarity to tdh2 gene/% |
| tdh1 | Vibrio parahaemolyticus | WP1 | Clinical | + | Chromosome | 97.0 |
| tdh2 | Vibrio parahaemolyticus | WP1 | Clinical | + | Chromosome | 100 |
| tdh3 | Vibrio parahaemolyticus | AQ3776 | Clinical | – | Chromosome | 98.6 |
| tdh4 | Vibrio parahaemolyticus | AQ3776 | Clinical | – | Chromosome | 98.6 |
| tdh5 | Vibrio parahaemolyticus | AQ3860 | Clinical | – | Plasmid | 98.9 |
| Vh-tdh | Vibrio hollisae | 9041 | Clinical | NT | Chromosome | 93.3 |
| NAG-tdh | Vibrio cholerae non-O1 | 91 | Clinical | NT | Plasmid | 98.6 |
| Vm-tdh | Vibrio mimicus | 6 | Clinical | NT | Chromosome | 97.0 |
| a: KP, beta-hemolysis on Wagatsuma agar unless otherwise specified. +: positive; –: negative; NT: not tested. | ||||||
表选项
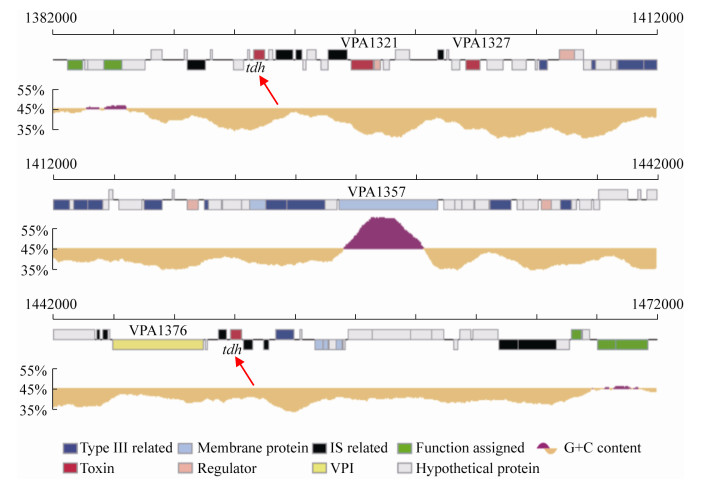 |
| 图 1 携带tdh基因的副溶血性弧菌2号染色体致病岛区域图示[10] Figure 1 Representation of the pathogenic island region of chromosome 2 of Vibrio parahaemolyticus carrying tdh gene[10]. |
| 图选项 |
2 tdh基因的多样性 典型的KP阳性表型的菌株都携带两个tdh基因(称为tdh1和tdh2),tdh1位于1.3 kb的Hind Ⅲ片段内;tdh2位于2.5 kb的Hind Ⅲ片段内[13]。而KP弱阳性或阴性表型的菌株却只含有单个tdh基因(称为tdh3),tdh3位于2.8 kb的Hind Ⅲ片段内[13]。此外还有一株十分特别的KP弱阳性菌株AQ3776,通过使用Hind Ⅲ限制性内切酶和凝胶电泳实验发现该菌株除了拥有tdh3外,还拥有由质粒介导位于5.0 kb Hind Ⅲ片段上的tdh基因,命名为tdh4[13]。这4种tdh基因拷贝编码的多肽均由189个氨基酸残基组成,包括165个氨基酸残基编码的成熟蛋白(分子量为18.5 kDa)和24个氨基酸残基编码的信号肽,其中173个氨基酸残基完全相同[14]。不仅如此,它们彼此间的基因序列也极其相似,tdh2与tdh1、tdh3、tdh4的序列相似度分别为97.0%、98.6%、98.6% (表 1) [9, 13]。通过SDS-PAGE实验发现4种tdh基因产物的迁移率十分接近,这也充分验证了它们之间的分子量和分子结构十分相似;通过刺激兔皮肤的血管通透性实验发现4种tdh基因产物的生物学活性完全一样,免疫学上无法区分[15]。所有tdh基因的编码区大小均为567 bp,tdh基因家族之间在编码区仅仅只有4–12个碱基差异,主要差异在于起始区、SD区和3′末端[14]。将4种tdh基因克隆到大肠杆菌中均可表达溶血活性,但除了tdh2外,其他几种的tdh基因只表达微弱的溶血活性[15]。通过构建tdh1突变体菌株进行实验发现,KP阳性菌株产生的溶血毒素90%以上都是由tdh2转录表达的,tdh1对溶血毒素的产生贡献不到10%[13]。tdh2与其他几种tdh基因在启动区存在较大差异,它相对于另外几种tdh基因而言具有很强的转录活性[13]。有研究表明,将KP阴性表型菌株tdh的启动子第134位(tdh基因启动子的敏感位点)的碱基替换掉可以大大增加tdh基因的表达至KP阳性菌株水平[16]。另外从一株KP阴性表型菌株AQ3860中还克隆出一种tdh基因,该基因位于1.0 kb的Hind Ⅲ片段上,命名为tdh5,同时该菌株也携带耐热性相关溶血素基因(trh),并以非常低的水平产生TDH[17]。tdh5在基因结构上与trh基因的关系比其他4种tdh基因与trh的关系更为密切,但它与tdh2的序列相似度依旧高达98.9%,因此tdh5有可能是tdh基因与trh基因之间转化的中间体[17]。
这些结果说明了tdh基因家族在基因结构上是高度同源性的,可能它们来源于同一祖先,之后经历了碱基突变才分化成一系列彼此之间存在微小差异的tdh基因,转录控制的差异是溶血表型中所见差异的主要原因[13, 16]。因此,KP阴性菌株也可能具有潜在的致病性,因为它们可能通过启动子中的位点突变而变成具有致病性的KP阳性菌株,这对我们具有重要的预警意义。
3 TDH的表达调控及其影响因素 所有的KP阳性菌株均具有tdh1和tdh2基因,且两者均在2号染色体80 kb致病岛Vp-PAI中编码[10],tdh2基因的表达是产生KP阳性现象的主要原因[13]。跨膜调节蛋白ToxR普遍存在于弧菌属中,操纵子ctx的转录取决于跨膜调节蛋白ToxR,而ctx直接影响tdh2的表达[18-20]。ToxR的活性被另一种跨膜蛋白ToxS进一步增强,并且编码ToxR和ToxS的基因(toxR和toxS)聚集在操纵子中[18]。另外,除ctx操纵子外,其他几种与毒力相关的基因也受到ToxR的协调调控。副溶血性弧菌的所有临床和环境菌株都具有Vp-toxRS基因,在存在Vp-ToxS的情况下,Vp-ToxR间接促进tdh2基因的表达[18]。
此外,研究人员还发现两种新型转录调节蛋白VtrA和VtrB调节致病岛Vp-PAI区域内编码的基因的表达,包括TDH和T3SS等相关毒力蛋白,VtrB的表达受VtrA的控制[21] (图 2)。vtrA或vtrB缺失菌株的全基因组转录分析表明,在这些缺失突变菌株中,超过60个基因的表达水平显著下调,并且这些基因几乎完全位于Vp-PAI区,这些结果说明了VtrA和VtrB是Vp-PAI毒力岛中毒力基因表达的主要调节剂,并且在副溶血性弧菌的致病性中起关键作用[21]。除了发现一些基因促进TDH的表达外还发现一些基因能够抑制TDH表达:RNA伴侣蛋白Hfq会在转录层面上抑制TDH等毒力相关因子的表达,在Hfq缺失突变体中TDH的产量远高于非突变菌株[22]。CalR是一种调节蛋白,最初被认为是副溶血性弧菌中群体运动和T3SS1基因表达的阻遏物,CalR结合于tdh2上游42 bp的转录起始位点,导致转录活性被抑制[23]。此外,KP测试结果显示副溶血性弧菌的溶血活性也受CalR的负调控,CalR还以直接方式抑制ToxR及其自身基因的表达[23-24]。不仅如此,群体感应基因luxM和luxS也参与副溶血弧菌毒力基因的表达,TDH受luxM负调控,受luxS正调控[25]。此外,一些环境因素也影响着TDH的表达。例如胆汁酸可以增强TDH的合成,含胆汁酸的环境有利于副溶血性弧菌的毒株的生长,并且胆汁酸还能增强其他多种毒力因子的表达[26]。例如胆汁酸能诱导VtrA介导的VtrB转录,胆汁反应性基因的转录组分析显示胆汁以VtrA依赖性方式强烈诱导Vp-PAI基因的表达,通过用胆汁酸螯合剂消胆胺处理胆汁酸后诱导活性降低[27]。胆汁酸还会使ToxR不稳定,从而增加与ToxS的相互作用,进一步促进ToxR的完全活性,从而正向调控TDH的表达分泌[28]。这也许是临床菌株携带tdh基因的概率远远大于环境菌株的一个重要原因(人体胃液中含有胆汁酸成分)。此外,温度与pH值均会影响TDH的表达调控[29-30]。例如副溶血性弧菌在25 ℃下诱导tdh的表达水平显著高于4 ℃和37 ℃;在低pH下使用小茴香精油和乳酸链球菌肽会导致副溶血弧菌产生的TDH毒素降低。
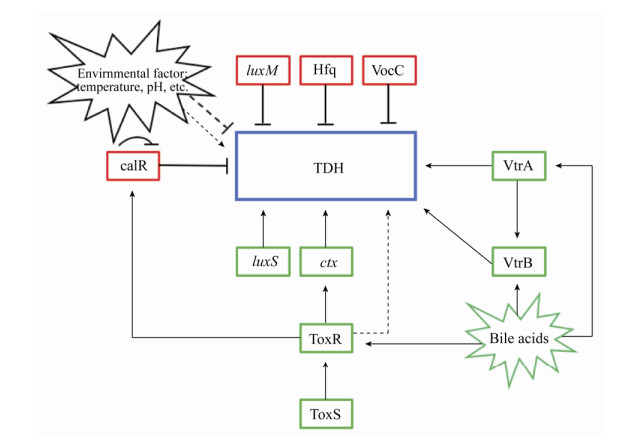 |
| 图 2 副溶血性弧菌TDH的表达调控示意图 Figure 2 Regulation of TDH in Vibrio parahaemolyticus. Arrows are positive regulation (red); and T bars are repression (green); dashed line is indirect regulation. |
| 图选项 |
简而言之,这些研究结果表明,副溶血性弧菌的tdh基因的表达受各种因素的严格调控,包括转录调节因子和生长条件及外界环境因素,深入了解影响tdh基因表达调控的因素有助于进一步研究副溶血性弧菌致病机理及靶向药物的开发。
4 TDH的蛋白结构 最新的一项研究还表明,TDH不仅通过一般分泌途径(Sec途径)分泌表达,而且一部分TDH在周质空间加工成熟后会返回细胞质,被T3SS2的分子伴侣蛋白VocC识别,从而通过T3SS2分泌途径分泌表达其肠毒性(图 3)[31]。TDH是一种非典型的成孔毒素,与细胞膜结合之前在溶液中以扁圆形的四聚体形式存在[32-33]。TDH四聚体形成中心孔,其直径为23 ? ,深度约为50 ? (图 4-A)[32]。组成TDH四聚体的启动子之间的π-阳离子相互作用对于TDH的溶血活性是必不可少的[32]。分子动力学模拟表明水分子可以通过中央和侧面通道自由渗透;电子显微照片显示TDH四聚体附着于脂质体,并且一些四聚体通过一个前体与脂质体结合[32]。TDH单体是一种β-夹层结构(β-sandwich structure),由10条β链和两个α螺旋(α1和310)组成,在cys151 (属于β10链)和cys161 (属于310螺旋)之间还存在一个分子内二硫键(图 4-B)[32]。此外,TDH还是一种耐热且可逆的淀粉样蛋白毒素,可根据温度变化调整其蛋白结构[34]。通过光谱和电子显微镜技术分析在各种热处理条件下TDH的结构时发现,当温度低于50 ℃时,TDH主要为由β折叠结构组成的天然寡聚肽;当温度升高到了60 ℃时,TDH转化为无毒的富含β链的纤维聚集体;当TDH被加热到90 ℃时,无论TDH在加热之前是天然结构还是纤维结构,它都会完全展开成未折叠状态(蛋白质可逆变性),冷却至37 ℃时会重新形成具有溶血活性的天然寡聚肽,通过添加刚果红(一种已知对淀粉样蛋白原纤维敏感的染料)可以抑制TDH的溶血活性[34]。
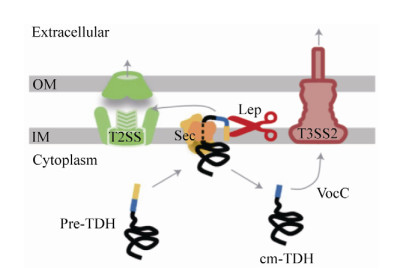 |
| 图 3 副溶血性弧菌中TDH分泌途径的模式图[31] Figure 3 A working model of the secretory pathways for TDH in V.? parahaemolyticus[31]. The precursor TDH with an N-terminal signal peptide (shown in yellow, called SP) is secreted by the Sec pathway, during the secretion process, SP is partially returned to the cytoplasm after being cut by Lep (lead peptidase) and secreted by the T3SS2 pathway under the action of the chaperone protein VocC. OM: outer membrane; IM: inner membrane. |
| 图选项 |
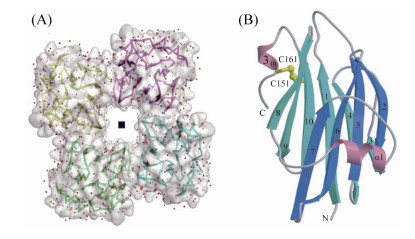 |
| 图 4 副溶血性弧菌TDH的蛋白结构[32] Figure 4 Protein structure of Vibrio parahaemolyticus TDH[32]. A: TDH tetramer structure; B: TDH monomer band structure (yellow represents molecular disulfide bond). |
| 图选项 |
维持具有中心孔的四聚体结构对于TDH的溶血活性是必需的,该结构一旦改变或者破坏,TDH便会丧失其溶血活性。因此,通过合成干扰或者破坏TDH蛋白结构的新型药物也许是未来对抗副溶血性弧菌感染病症的一个方向。
5 TDH的生物活性 早期研究表明,TDH对人的红细胞及各种哺乳动物(马的红细胞除外)均具有很高的溶血活性,GT1神经节苷脂是TDH与红细胞膜结合产生溶血活性的主要受体位点[35]。TDH在红细胞膜的磷脂双分子层中形成跨膜孔,可渗透水和离子自由通过,由于细胞膜内外的离子不平衡致使膜透化和细胞膨胀,最终导致红细胞裂解破裂[36]。
TDH还对各种培养细胞具有细胞毒性,如Hela细胞、L细胞[37]、FL细胞[38]和源自人肠的CCL-6细胞[39]等。通过显微镜观察用TDH处理过的细胞发现,细胞表面的绒毛从有到无,细胞质逐渐变性,细胞核崩解直至细胞完全裂解[38]。TDH可以从细胞外部和内部诱导其细胞毒性,并通过细胞凋亡杀死细胞[40]。TDH的细胞毒性依赖于脂筏,细胞鞘磷脂的外源性和内源性消耗也阻止了TDH细胞毒性,但是用脂质覆盖测定或脂质体吸收测试未检测到TDH和鞘磷脂之间的直接相互作用,因此鞘磷脂对于TDH与脂筏的结合是极其重要的,但它却不是TDH的分子靶标[41]。TDH诱导细胞内Ga2+的快速瞬时增加以及通过降低细胞周期的速率也对其细胞毒性发挥着关键作用[42]。此外TDH还可以下调结肠癌细胞的增殖,并在其作用机制中涉及CaSR。下调主要通过E-钙粘蛋白和β-连环蛋白介导途径的参与和细胞周期调节剂的抑制以及细胞周期抑制剂的上调而产生作用[43]。通过结合表皮生长因子受体(EGFR)结合肽和TDH的R46E突变制备新型特异性免疫毒素,TDH的R46E突变与靶亲和试剂的结合为癌症的药理学治疗开辟了新的可能性并增强了抗癌药物的作用[44]。
同时,TDH还具有诱导小肠产生肠毒素的作用,这也是造成炎症性腹泻的主要原因[45-46]。Ca2+似乎是参与刺激肠道分泌的重要信使[45],通过显微荧光测定法发现,TDH在Caco-2 (人结肠上皮细胞)和IEC-6细胞(大鼠小肠上皮细胞)中引起细胞内游离Ga2+依赖性增加,只有在低TDH浓度时,这种效应才是可逆的,并且TDH激活的离子流入途径对Ga2+没有选择性[46]。此外,在相同浓度范围内,TDH促进Ga2+依赖的Cl-的分泌。高浓度时,通过功能、生化和形态学分析,TDH可诱导剂量依赖但不依赖Ga2+的细胞死亡[46]。
因此可以得出结论,TDH是副溶血性弧菌的主要的毒力因子之一,发挥着几种主要的生物活性,包括溶血活性、细胞毒性和肠毒性等。进一步研究影响调控TDH生物活性表达的因素和致病机理有利于更好地应对致病性副溶血性弧菌的感染。此外,这些生物活性的表达往往还有其他毒力因子的参与,比如致病岛上的Ⅲ型分泌系统等,TDH与其他毒力因子协同作用的调控机制也是今后亟需解决的一个研究方向。
6 结论和展望 TDH是副溶血性弧菌最主要的毒力因子之一。从TDH的发现至今经历了几十年的时间,国内外****对这种毒力因子进行了广泛深入的研究,但仍存在很多问题有待深入探索,如TDH与其他毒力因子联合作用的致病机理,环境因素对TDH分泌表达的潜在影响以及tdh基因在弧菌属中的起源等。
本实验室对大量的临床/环境副溶血性弧菌进行了普查,发现不仅绝大多数的临床菌株携带tdh基因,而且携带tdh基因的环境菌株占比也很高[47]。近年来,越来越多的报道发现环境菌株携带tdh基因的占比在增加[48-50],这可能会进一步加大水产养殖动物患病的几率以及人类被感染的风险。环境菌株携带tdh基因的发生率与样品的来源或位置有关,一般来说温度越高,检出率就越高[49, 51]。此外,携带tdh基因的菌株的生长异质性与生物被膜形成能力都显著大于未携带tdh基因的菌株[52-53]。我们发现,携带tdh或相关基因的菌株耐药性远远大于不带tdh基因的菌株[54]。这些结果表明tdh基因可能具有其他作用,例如有助于自然竞争,增加自身的存活率;抵抗外界环境刺激,特别是携带tdh基因的环境菌株可能通过调控毒力因子的表达进而更稳定的定殖在宿主身上;在选择性压力下促进耐药基因表达等。此外,TDH还有很多其他潜在功能待深入研究:(1) TDH在提升副溶血性弧菌菌株自然竞争力中的作用及其相关机制;(2)携带tdh基因的环境菌株与临床菌株之间在表型和基因型上的差异;(3) tdh基因转录的详细调控机制与蛋白分泌途径,以及外界环境刺激与产生TDH之间的潜在关联。深入研究这些科学问题可为基因工程疫苗与新型抗生素及靶向药物的开发、制定有效的疾病防控策略提供新的方法和思路。
References
| [1] | Lopatek M, Wieczorek K, Osek J. Antimicrobial Resistance, Virulence Factors, and Genetic Profiles of Vibrio parahaemolyticus from Seafood. Applied and Environmental Microbiology, 2018, 84(16). |
| [2] | Mu LL, NIU B, Zhao Y. Advances in research on the role of Vibrio parahaemolyticus secretion system in pathogenicity. Acta Microbiologica Sinica, 2019, 59(4): 37-47. (in Chinese) 穆丽丽, 牛犇, 赵勇. 副溶血性弧菌分泌系统在致病力中作用的研究进展. 微生物学报, 2019, 59(4): 37-47. |
| [3] | Li Y, Zhang S, Li J, Chen ML, He M, Wang YY, Zhang YC, Jing HB, Ma HM, Li YD, Zhao L, Zhao HQ, Kan B, Pang B. Application of digital PCR and next generation sequencing in the etiology investigation of a foodborne disease outbreak caused by Vibrio parahaemolyticus. Food microbiology, 2019, 84: 103233. |
| [4] | Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J. Vibrio spp.infections. Nature Reviews Disease Primers, 2018, 4: 8. |
| [5] | 赵永刚.副溶血弧菌tdh、trh和tlh基因的克隆、表达及基因敲除对其溶血活性的影响.中国海洋大学博士学位论文, 2010. |
| [6] | Baba K, Shirai H, Terai A, Kumagai K, Takeda Y, Nishibuchi M. Similarity of the tdh gene-bearing plasmids of Vibrio cholerae non-O1 and Vibrio parahaemolyticus. Microb Pathog, 1991, 10(1): 61-70. |
| [7] | Terai A, Shirai H, Yoshida O, Takeda Y, Nishibuchi M. Nucleotide sequence of the thermostable direct hemolysin gene (tdh gene) of Vibrio mimicus and its evolutionary relationship with the tdh genes of Vibrio parahaemolyticus. FEMS Microbiology Letters, 1990, 71(3): 319-323. |
| [8] | Yamasaki S, Shirai H, Takeda Y, Nishibuchi M. Analysis of the gene of Vibrio hollisae encoding the hemolysin similar to the thermostable direct hemolysin of Vibrio parahaemolyticus. FEMS Microbiology Letters, 1991, 80(2/3): 259-263. |
| [9] | Nishibuchi M, Kaper JB. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infection and Immunity, 1995, 63(6): 2093-2099. |
| [10] | Makino K, Oshima K, Kurokawa K, Yokoyama K, Uda T, Tagomori K, Iijima Y, Najima M, Nakano M, Yamashita A, Kubota Y, Kimura S, Yasunaga T, Honda T, Shinagawa H, Hattori M, Iida T. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae.. The Lancet, 2003, 361(9359): 743-749. |
| [11] | Groisman EA, Ochman H. Pathogenicity Islands: Bacterial Evolution in Quantum Leaps. Cell, 1996, 87(5): 791-794. |
| [12] | Terai A, Baba K, Shirai H, Yoshida O, Takeda Y, Nishibuchi M. Evidence for insertion sequence-mediated spread of the thermostable direct hemolysin gene among Vibrio species. Journal of Bacteriology, 1991, 173(16): 5036-5046. |
| [13] | Nishibuchi M, Kaper JB. Duplication and variation of the thermostable direct haemolysin (tdh) gene in Vibrio parahaemolyticus. Molecular Microbiology, 1990, 4(1): 87-99. |
| [14] | Taniguchi H, Hirano H, Kubomura S, Higashi K, Mizuguchi Y. Comparison of the nucleotide sequences of the genes for the thermostable direct hemolysin and the thermolabile hemolysin from Vibrio parahaemolyticus. Microbial Pathogenesis, 1986, 1(5): 425-432. |
| [15] | Yoh M, Honda T, Miwatani T, Nishibuchi M. Characterization of thermostable direct hemolysins encoded by four representative tdh genes of Vibrio parahaemolyticus. Microbial pathogenesis, 1991, 10(2): 165-172. |
| [16] | Okuda J, Nishibuchi M. Manifestation of the Kanagawa phenomenon, the virulence-associated phenotype, of Vibrio parahaemolyticus depends on a particular single base change in the promoter of the thermostable direct haemolysin gene. Molecular Microbiology, 1998, 30(3): 499-511. |
| [17] | Baba K, Shirai H, Terai A, Nishibuchi M. Analysis of the tdh gene cloned from a tdh gene- and trh gene- positive strain of Vibrio parahaemolyticus. Microbiology and Immunology, 1991, 35(3): 253-258. |
| [18] | Lin Z, Kumagai K, Baba K, Mekalanos JJ, Nishibuchi M. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. Journal of Bacteriology, 1993, 175(12): 3844-3855. |
| [19] | Miller VL, Mekalanos JJ. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proceedings of the National Academy of Sciences of the United States of America, 1984, 81(11): 3471-3475. |
| [20] | Miller VL, Taylor RK, Mekalanos JJ. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell, 1987, 48(2): 271-279. |
| [21] | Kodama T, Gotoh K, Hiyoshi H, Morita M, Izutsu K, Akeda Y, Park KS, Cantarelli VV, Dryselius R, Iida T, Honda T. Two regulators of Vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region. PLoS ONE, 2010, 5(1): e8678. |
| [22] | Nakano M, Takahashi A, Su Z, Harada N, Mawatari K, Nakaya Y. Hfq regulates the expression of the thermostable direct hemolysin gene in Vibrio parahaemolyticus. BMC Microbiology, 2008, 8(1): 155. |
| [23] | Zhang Y, Zhang Y, Gao H, Zhang L, Yin Z, Huang X, Zhou D, Yang H, Yang W, Wang L. Vibrio parahaemolyticus, CalR down regulates the thermostable direct hemolysin (TDH) gene transcription and thereby inhibits hemolytic activity. Gene, 2017, 613: 39-44. |
| [24] | Osei-Adjei G, Gao H, Zhang Y, Zhang L, Yang W, Yang H, Yin Z, Huang X, Zhang Y, Zhou D. Regulatory actions of ToxR and CalR on their own genes and type Ⅲ secretion system 1 in Vibrio parahaemolyticus. Oncotarget, 2017, 8(39): 65809-65822. |
| [25] | Guo M, Fang Z, Sun L, Sun D, Wang Y, Li C, Wang R, Liu Y, Hu H, Liu Y, Xu D, Gooneratne R. Regulation of Thermostable direct hemolysin and biofilm formation of Vibrio parahaemolyticus by Quorum-Sensing genes luxM and luxS. Current Microbiology, 2018, 75(9): 1190-1197. |
| [26] | Pace JL, Chai TJ, Rossi HA, Jiang X. Effect of bile on Vibrio parahaemolyticus. Applied and Environmental Microbiology, 1997, 63(6): 2372-2377. |
| [27] | Gotoh K, Kodama T, Hiyoshi H, Izutsu K, Park KS, Dryselius R, Akeda Y, Honda T, Iida T. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS ONE, 2010, 5(10): e13365-. |
| [28] | Midgett CR, Almagro-Moreno S, Pellegrini M, Taylor RK, Skorupski K, Kull FJ. Bile salts and alkaline pH reciprocally modulate the interaction between the periplasmic domains of Vibrio cholerae ToxR and ToxS. Molecular Microbiology, 2017, 105(2): 258-272. |
| [29] | Feng B, Guo Z, Zhang W, Pan Y, Zhao Y. Metabolome response to temperature-induced virulence gene expression in two genotypes of pathogenic Vibrio parahaemolyticus. BMC Microbiology, 2016, 16(1): 75. |
| [30] | Mahoney JC, Gerding MJ, Jones SH, Whistler CA. Comparison of the pathogenic potentials of environmental and clinical Vibrio parahaemolyticus strains indicates a role for temperature regulation in virulence. Applied and Environmental Microbiology, 2010, 76(22): 7459-7465. |
| [31] | Matsuda S, Okada R, Tandhavanant S, Hiyoshi H, Gotoh K, Iida T, Kodama T. Export of a Vibrio parahaemolyticus toxin by the Sec and type Ⅲ secretion machineries in tandem. Nature Microbiology, 2019, 4(5): 781-788. |
| [32] | Yanagihara I, Nakahira K, Yamane T, Kaieda S, Mayanagi K, Hamada D, Fukui T, Ohnishi K, Kajiyama S, Shimizu T, Sato M, Ikegami T, Ikeguchi M, Honda T, Hashimoto H. Structure and functional characterization of Vibrio parahaemolyticus thermostable direct hemolysin. Journal of Biological Chemistry, 2010, 285(21): 16267-16274. |
| [33] | Kundu N, Tichkule S, Pandit SB, Chattopadhyay K. Disulphide bond restrains the C-terminal region of thermostable direct hemolysin during folding to promote oligomerization. Biochemical Journal, 2017, 474(2): 317-331. |
| [34] | Cai Q, Zhang Y. Structure, function and regulation of the thermostable direct hemolysin (TDH) in pandemic, Vibrio parahaemolyticus. Microbial Pathogenesis, 2018, 123: 242-245. |
| [35] | Takeda Y, Takeda T, Honda T, Miwatani T. Inactivation of the biological activities of the thermostable direct hemolysin of Vibrio parahaemolyticus by ganglioside Gt1. Infection and Immunity, 1976, 14(1): 1-5. |
| [36] | Lang PA, Kaiser S, Myssina S, Birka C, Weinstock C, Northoff H, Wieder T, Lang F, Huber SM. Effect of Vibrio parahaemolyticus haemolysin on human erythrocytes. Cellular Microbiology, 2004, 6(4): 391-400. |
| [37] | Sakazaki R, Tamura K, Nakamura A, Kurata T, Goda A. Studies on enteropathogenic activity of Vibrio parahaemolyticus using ligated gut loop model in rabbits. Japanese Journal of Medical Science & Biology, 1974, 27(1): 35-43. |
| [38] | Sakurai J, Honda T, Jinguji Y, Arita M, Miwatani T. Cytotoxic effect of the thermostable direct hemolysin produced by Vibrio parahaemolyticus on FL cells. Infection and Immunity, 1976, 13(3): 876-883. |
| [39] | Honda T, Tekada Y, Miwatani T. Pathogenesis of Vibrio Parahaemolyticus. Natural Toxins, 1980: 251-258. |
| [40] | Naim R, Yanagihara I, Iida T, Honda T. Vibrio parahaemolyticus thermostable direct hemolysin can induce an apoptotic cell death in Rat-1 cells from inside and ouside of the cells. FEMS Microbiology Letters, 2001, 195(2): 237-244. |
| [41] | Matsuda S, Kodama T, Okada N, Okayama K, Honda T, Iida T. Association of Vibrio parahaemolyticus thermostable direct hemolysin with lipid rafts is essential for cytotoxicity but not hemolytic activity. Infection and Immunity, 2010, 78(2): 603-610. |
| [42] | Tang GQ, Iida T, Yamamoto K, Honda T. Ca2+-independent cytotoxicity of Vibrio parahaemolyticus thermostable direct hemolysin (TDH) on Intestine 407, a cell line derived from human embryonic intestine. FEMS Microbiology Letters, 1995, 134(2/3): 233-238. |
| [43] | Chowdhury P, Pore D, Mahata N, Karmakar P, Pal A, Chakrabarti MK. Thermostable direct hemolysin downregulates human colon carcinoma cell proliferation with the involvement of E-cadherin, and β-catenin/Tcf-4 signaling. PLoS ONE, 2011, 6(5): e20098. |
| [44] | Huang SC, Wang YK, Huang WT, Kuo TM, Yip BS, Li TH, Wu TK. Potential antitumor therapeutic application of Grimontia hollisae thermostable direct hemolysin mutants. Cancer Science, 2015, 106(4): 447-754. |
| [45] | Baffone W, Casaroli A, Campana R, Citterio B, Vittoria E, Pierfelici L, Donelli G. In vivo studies on the pathophysiological mechanism of Vibrio parahaemolyticus TDH+-induced secretion. Microbial Pathogenesis, 2005, 38(2/3): 133-137. |
| [46] | Raimondi F, Kao JP, Fiorentini C, Fabbri A, Donelli G, Gasparini N, Rubino A, Fasano A. Enterotoxicity and cytotoxicity of Vibrio parahaemolyticus thermostable direct hemolysin in in vitro systems. Infection and Immunity, 2000, 68(6): 3180-3185. |
| [47] | Song XY, Ma YJ, Fu JJ, Zhao AJ, Guo ZR, Malakar PK, Pan YJ, Zhao Y. Effect of temperature on pathogenic and non-pathogenic Vibrio parahaemolyticus biofilm formation. Food Control, 2017, 73: 485-591. |
| [48] | Raghunath P, Karunasagar I, Karunasagar I. Improved isolation and detection of pathogenic Vibrio parahaemolyticus from seafood using a new enrichment broth. International Journal of Food Microbiology, 2009, 129(2): 200-203. |
| [49] | Raghunath, P. Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Frontiers in Microbiology, 2015, 5: 805. |
| [50] | Baliga P, Shekar M, Ahamed ST, Venugopal, M N. Antibiotic resistance pattern and its correlation to the presence of tdh gene and CRISPR-Cas system in Vibrio parahaemolyticus strains isolated from seafood. Indian Journal of Fisheries, 2019, 66(2): 101-108. |
| [51] | Flynn A, Davis BJK, Atherly D, Olson G, Bowers JC, DePaola A, Curriero FC. Associations of Environmental Conditions and Vibrio parahaemolyticus Genetic Markers in Washington State Pacific Oysters. Frontiers in Microbiology, 2019, 10: 2797. |
| [52] | Liu BX, Liu HQ, Pan YJ, Zhao Y. Comparison of the effects of environmental parameters on the growth variability of Vibrio parahaemolyticus coupled with strain sources and genotypes analyses. Frontiers in Microbiology, 2016, 7: 994. |
| [53] | Zhao AJ, Fu JJ, Song XY, Sun XH, Pan YJ, Zhao Y. Analysis of biofilm formation on pathogenic and non-pathogenic Vibrio parahaemolyticus at different temperatures and contact materials. Journal of Food Science and Biotechnology, 2018, 37(1): 7-14. (in Chinese) 赵爱静, 付娇娇, 宋雪迎, 孙晓红, 潘迎捷, 赵勇. 致病性与非致病性副溶血性弧菌在不同温度和接触材料表面生物被膜形成情况分析. 食品与生物技术学报, 2018, 37(1): 7-14. |
| [54] | Li H, Tang R, Lou Y, Cui ZC, Chen WJ, Hong Q, Zhang ZH, Malakar PK, Pan YJ, Zhao Y. A comprehensive epidemiological research for clinical Vibrio parahaemolyticus in Shanghai. Frontiers in Microbiology, 2017, 8: 1043. |
