汤芳1, 潘子豪1, 李德志1, 马琳2, 熊毅2, 陆承平1


1. 南京农业大学, 世界动物卫生组织猪链球菌病参考实验室, 农业部动物细菌学重点实验室, 江苏 南京 210095;
2. 广西壮族自治区动物疫病预防控制中心, 广西 南宁 530001
收稿日期: 2015-05-29; 修回日期: 2015-09-01; 网络出版日期: 2015-09-11
基金项目: 国家自然科学基金(31402213),江苏省自然科学基金(BK20140686),中央高校基本科研业务费专项资金资助(KJQN201525)
通信作者: 陆承平, Tel/Fax: +86-25-84396517;E-mail: lucp@njau.edu.cn
摘要: [目的] 猪链球菌是一种能感染人和猪的人畜共患病病原,并且还可零星感染多种哺乳动物。本试验旨在调查流浪猫携带猪链球菌的情况。[方法] 在流浪猫身上分离猪链球菌,经血清凝集实验和PCR检测,鉴定其血清型;经多序列位点分型分析,鉴定其ST型;将所分离的细菌与GenBank上已公布的猪链球菌构建16S rRNA的系统发育树,分析该菌株与其他猪链球菌的亲缘关系;药敏纸片法分析其耐药性;小鼠攻毒试验分析其毒力。[结果] 本试验在流浪猫身上分离到一株猪链球菌,命名为m70,其血清型为9型。多序列位点分型显示,m70株属于一个新的ST型。与GenBank上已公布的猪链球菌16S rRNA进行系统发育树分析,结果显示m70属于一个单独的分支。m70与临床菌株的耐药情况相似,对四环素耐药,对红霉素中介耐药,对氨苄西林敏感。小鼠攻毒试验显示,感染108 CFU剂量m70的小鼠,死亡率达到60%-80% (3/5-4/5),3次攻毒试验的平均LD50为5.1×107 CFU;而本实验室保存的猪链球菌强毒株HA9801感染小鼠的平均LD50为3.9×107 CFU,两者之间没有显著差异(P <0.05)。[结论] 从流浪猫身上分离得到的猪链球菌m70属于优势血清型,且毒力较强,提示一些流行血清型的猪链球菌强毒株具有从流浪猫传染人的潜在风险。
关键词: 猪链球菌血清型9型野猫ST型抗生素抗性小鼠
Isolation and characterization of a Streptococcus suis serotype 9 from a wild cat
Fang Tang1, Zihao Pan1, Dezhi Li1, Lin Ma2, Yi Xiong2, Chengping Lu1


1. OIE Reference Laboratory for Swine Streptococcosis, Key Laboratory of Animal Bacteriology, Ministry of Agriculture, College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China;
2. Guangxi Center for Animal Disease Control and Prevention, Nanning 530001, Guangxi Zhuang Autonomous Region, China
Received: 29 May 2015; Revised: 1 September 2015; Published online: 11 September 2015
Foundation Item: Supported by the National Natural Science Foundation of China (31402213), by the Natural Science Foundation of Jiangsu Province (BK20140686) and by the Fundamental Research Funds for the Central Universities (KJQN201525)
Corresponding author: Chengping Lu, Tel/Fax: +86-25-84396517; E-mail: lucp@njau.edu.cn
Abstract:[Objective] Streptococcus suis (S. suis) is an emerging zoonotic pathogenic bacterium capable of infecting piglets and human and with sporadic infections in a variety of mammalian species. The aim of this study is to investigate the prevalence of S. suis in wild cats. [Methods] We isolated an S. suis strain from a wild cat. We tested the serotype of the isolated strain by anti-serum agglutination and PCR. We determined the sequence type (ST) of the isolated strain by multilocus sequence typing tests (MLST). We constructed the 16S rRNA phylogenetic tree of the isolation and S. suis strains in NCBI database to demonstrated genetic relationship of different strains. We measured the antibiotic resistance of the isolated strain by triple disk diffusion method. We detected the virulence of the isolated strain by mice infection experiments. [Results] We isolated an S. suis strain m70 from a wild cat, which belongs to serotype 9. MLST showed that m70 fell into a new ST. The 16S rRNA phylogenetic tree of m70 and S. suis strains in NCBI database demonstrated that m70 was in a separate cluster. m70 was resistant to tetracycline, intermediate to erythromycin, and sensitive to ampicillin, corresponding to clinical S. suis isolates in China. The mortality of mice infected with 108 CFU of m70 was achieved 60%-80% (3/5-4/5). The mean LD50 of mice infected with m70 was 5.1×107 CFU, while the mean LD50 of virulent S. suis strain HA9801 was 3.9×107 CFU. There is no significant difference between the LD50 of the two strains(P<0.05). [Conclusion] We isolated an S. suis strain from a wild cat, which belongs to the prevalent serotype and was a virulent strain, indicating the potential of transmission of S. suis from wild cats to humans, especially some prevalent serotype strains.
Key words: Streptococcus suisserotype 9wild catST typeantibiotic resistancemice
猪链球菌是一种能引起猪的脑膜炎、关节炎和败血症的致病菌,并可感染人[1, 2]。根据猪链球菌荚膜抗原的多样性,可将猪链球菌分为33个血清型(1-31、33和1/2)[3, 4]。1998年,江苏爆发了一场猪链球菌病,病原主要为猪链球菌2型,造成了25例感染[5]。2005年7月,四川又出现了一次大爆发,多达215人感染,且还伴有其他省市的零星感染[6, 7, 8]。到2012年底,文献报道的猪链球菌感染的案例多达1584起,其中大部分是感染猪和人的案例[9]。猪链球菌还可造成多种哺乳动物的零星感染,如家牛、野牛、马、绵羊、山羊、家禽和野猪[10, 11, 12, 13, 14]。据我们所知,尚未有猪链球菌感染流浪猫的报道。本研究中,我们从广西的流浪猫身上分离到一株猪链球菌,在国内尚属首次。
1 材料和方法 1.1 主要仪器和试剂PCR仪购自日本TaKaRa公司;分光光度计购自美国Bio-Rad公司;THB (Todd-HewittBroth)培养基购自英国Oxoid公司;MHA(Mueller-Hinton Agar)培养基购自英国Oxoid公司;基因组提取试剂盒购自美国Omega公司;胶回收试剂盒购自TaKaRa公司;PCR mix购自日本TaKaRa公司;药敏纸片购自杭州微生物试剂有限公司;猪链球菌33种标准血清由中国动物卫生与流行病学中心惠赠。
1.2 细菌的分离与鉴定从广西无明显症状的流浪猫身上采集246份肝脏和肺脏的样品,将样品在75%的酒精中浸泡15 min消毒,随后用无菌剪刀在样品上剪开一小口,用无菌接种环在剪开的小口内蘸取样品,划线接种于THB平板,然后将平板倒置于37 °C温箱培养过夜。次日挑取疑似菌落放入液体THB中培养,并进行革兰氏染色鉴定。
1.3 猪链球菌的种特异性鉴定用基因组提取试剂盒提取m70的基因组DNA,以此为模板,以gdh基因特异性引物gdh-F和gdh-R[15](表1)扩增猪链球菌特异性片段。PCR程序为:94 °C 5 min;94 °C 1 min,55 °C 30 s,72 °C 1 min,30个循环;72 °C 10 min。以m70基因组DNA为模板,以16S rRNA通用引物[16] 16S rRNA -F和16S rRNA -R(表1)扩增16S rRNA特异性条带。PCR程序为:94 °C 5 min;94 °C 1 min,55 °C 30 s,72 °C 1.5 min,30个循环;72 °C 10 min。PCR产物用1%琼脂糖凝胶进行电泳,再用胶回收试剂盒回收特异性条带,并送公司测序,测序结果在GenBank上用BLAST进行比对。并用MAGE 5.1的Neighbor-Joining Algorithm[17]方法对m70和NCBI上已公布的18株猪链球菌的16S rRNA的序列构建系统发育树。
表 1. 扩增目的基因及引物序列Table 1. Primers of target genes
| Genes | Primers | Primer sequences (5'→3') | Product sizes/bp |
| gdh | gdh-F | CCATGGACAGATAAAGATGG | 688 |
| gdh-R | GCAGCGTATTCTGTCAAACG | ||
| 16S rRNA | 16S rRNA-F | AGAGTTTGATCGTGGCTCA | 1500 |
| 16S rRNA-R | TACGGTTACCTTGTTACGACTT | ||
| cps9 | cps9-F | GATTCCAACGGAGGTTGTCT | 484 |
| cps9-R | CGATCCTGAAGCAATCATGC |
表选项
1.4 血清型鉴定用经典的血清凝集实验对m70的血清型进行鉴定。将m70培养至对数期,随后5000 r/min离心15 min,弃上清,菌体用灭菌生理盐水洗涤2遍,随后以同样体积重悬于灭菌生理盐水中。将菌体重悬液分别与猪链球菌的33种标准血清进行玻片凝集实验,观察是否出现凝集现象,生理盐水作为阴性对照。为进一步鉴定m70的血清型,又用猪链球菌9型特异性鉴定引物cps9-F和cps9-R[18]对m70 DNA进行了扩增,同时用猪链球菌9型菌株NJ-2的DNA和ddH2O作为阳性和阴性对照模板。PCR程序为:94 °C 5 min;94 °C 30 s,56 °C 30 s,72 °C 1 min,30个循环;最后72 °C 5 min。PCR产物用1%的琼脂糖凝胶进行电泳,再用胶回收试剂盒回收特异性条带,并送公司测序,测序结果在GenBank上用BLAST进行比对。
1.5 MLST 分型对m70进行多位点序列分型(Multiple locus sequence typing ,MLST)。根据MLST数据库(http://pubmlst.org/ssuis/)公布的7个看家基因(dpr、mutS、cpn60、thrA、recA、aroA和gki)的引物序列对m70的基因组DNA进行PCR扩增[19]。PCR产物用1%的琼脂糖凝胶进行电泳,再用胶回收试剂盒回收相应条带,回收产物送公司测序,所得序列到MLST数据库比对,进行序列型(sequence type,ST)分析。
1.6 药敏试验根据美国临床和实验室标准协会(Clinical and Laboratory Standards Institute,CLSI)[20]的标准进行药敏试验。用含有5%的小牛血清的MH培养基将m70培养至OD600 = 0.08-0.13 (相当于0.5麦氏浓度标准),用灭菌棉签将菌液均匀的涂布在MHA平板上,然后将氯霉素、氟苯尼考、红霉素、四环素、环丙沙星等13种药物(表2)的药敏纸片平均贴在涂有细菌的平板上,每块平板上最多贴5个药敏纸片,随后将平板倒置于37 °C温箱培养过夜,次日根据抑菌圈直径判断细菌对各药物的敏感性。
表 2. m70株对常用抗生素的敏感性Table 2. Sensitivity of m70 strain to several common antibiotics
| Antibiotics | Diameter of antibacterial circle/mm | Antibiotic resistance | Antibiotics | Diameter of antibacterial circle/mm | Antibiotic resistance |
| Erythromycin | 18 | I | Kanamycin | 12 | - |
| Chloromycetin | 27 | S | Amikacin | 13 | - |
| Ciprofloxacin | 19 | S | Ampicillin | 25 | S |
| Acheomycin | 11 | R | Ceftizoxime | 20 | - |
| Florfenicol | 27 | - | Cefotaxime | 26 | I |
| Gentamycin | 17 | - | Doxycycline | 25 | - |
| Enrofloxacin | 22 | S | |||
| S, sensitive; I, intermediate; R, resistance; -, no standard. | |||||
表选项
1.7 小鼠致病性试验参照陈涛[21]等的方法,将m70培养至对数期,5000 r/min离心10 min,弃上清,菌体用无菌PBS洗涤3次,然后将菌体用无菌PBS稀释到适当浓度。将25只BALB/c雌性小鼠随机分为5组,每组5只,每组腹腔注射一个梯度,分别为108、107、106和105 CFU (表3),空白组小鼠每只注射0.2 mL PBS。同时采取同样的方法用实验室保存的猪链球菌强毒株HA9801对BALB/c小鼠攻毒。攻毒后观察7 d,记录小鼠的死亡情况,试验重复3次。
表 3. m70和HA9801株对小鼠的致死率Table 3. Mortality induced by m70 and HA9801 in mice
| Dose (CFU) | Mortality of mice | ||||||
| m70 | HA9801 | ||||||
| Test 1 | Test 1 | Test 3 | Test 1 | Test 1 | Test 3 | ||
| 10 8 | 4/5 | 4/5 | 3/5 | 4/5 | 5/5 | 4/5 | |
| 10 7 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 10 6 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 10 5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| PBS | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| LD 50 | 4.3×10 7 | 4.3×10 7 | 6.8×10 7 | 4.3×10 7 | 3.2×10 7 | 4.3×10 7 | |
| Mean LD 50 | 5.1×10 7 | 3.9×10 7 | |||||
表选项
2 结果和分析 2.1 细菌的分离与鉴定显微镜下观察,m70株呈短链状的革兰氏阳性球菌(图1-A)。在血平板上形成草绿色α溶血环(图1-B)。用特异性引物扩增猪链球菌特异性基因gdh,在688 bp位置出现特异性条带,经测序比对,该序列与NCBI上猪链球菌2208株的gdh基因序列的同源性高达98% (675/688)。16S rRNA扩增也出现1500 bp的特异性条带,经测序比对,该序列与NCBI上猪链球菌的16S rRNA基因序列的同源性达到99% (1482/1490),由此确定m70为猪链球菌。m70和NCBI上其他猪链球菌的16S rRNA基因的系统发育树分析结果显示,m70株位于一个单独的分支上(图2),这可能与m70的宿主特异性相关。
 |
| 图 1. m70 形态照片 Figure 1. The morphology of m70. A: Gram staining of m70 strain; B: m70 cultured on sheep blood agar plate |
| 图选项 |
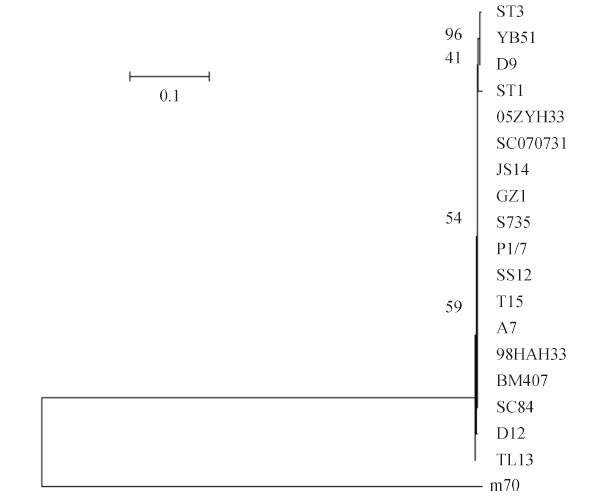 |
| 图 2. 基于猪链球菌16S rRNA的系统发育树 Figure 2. Phylogenetic tree based on the 16S rRNA genes of 19 strains of S. suis. Neighbor-joining tree analysis and bootstrap analysis (1000 replicates) based on the alignment of the DNA sequence of the 16S rRNA genes. The numbers at the nodes indicate the bootstrap probabilities of that particular branch. The coefficient is 0.1. |
| 图选项 |
2.2 血清型鉴定血清凝集试验结果显示,m70菌液与猪链球菌9型标准血清发生凝集反应,表明m70血清型为9型。为进一步确定其血清型,我们以9型特异性引物cps9对m70的DNA进行了PCR扩增,结果在484 bp位置出现特异性条带(图3),阳性对照NJ-2也扩增出484 bp的条带,而阴性对照则未扩增出条带,进一步证实m70血清型为9型。临床分离的致病性猪链球菌除2型外,9型也是一个流行的血清型[22]。 欧洲发达国家如澳大利亚、荷兰、比利时、德国等地频繁的从病猪身上分离到猪链球菌9型[23]。过去几年的流行病学调查结果显示,在中国,猪链球菌9型的流行也呈逐年上升的趋势[24]。猪链球菌在感染过程中并没有严格的宿主特异性,它可造成多种哺乳动物的零星感染,而流行血清型的菌株有更多的机会接触不同的宿主,因此本试验可在流浪猫身上分离到一株9型菌株,提示临床上流行血清型的菌株感染猪以外其他宿主的几率更高。
 |
| 图 3. m70株的cps9基因扩增电泳图 Figure 3. PCR amplification of the cps9 gene of m70 strain. M: marker; lane 1: NJ-2; lane 2: m70; lane 3: ddH2O. |
| 图选项 |
2.3 MLST 分型MLST分型结果显示,m70株属于一个新的ST型。据之前的报道,我国主要流行的ST型为ST7 和ST1[25, 26, 27, 28]。有意思的是,m70属于一个新的ST型,这与m70的16S rRNA系统发育树属于一个单独的分支的结果相对应。虽然m70属于一个流行的血清型9型,但它在16S rRNA系统发育树上属于一个单独的分支,ST分型上也属于一个新的型,这可能与它不寻常的宿主特异性相关。
2.4 药敏试验药敏结果显示,m70株对四环素耐药,对红霉素中介耐药,对氨苄西林、环丙沙星、氯霉素和恩诺沙星敏感(表2)。世界范围内猪链球菌临床分离株普遍对大环内酯类和四环素类耐药,对大环内脂类的耐药率高达70%,而对四环素类耐药率高达90%[29]。由于四环素类和大环内脂类抗生素被广泛用于临床猪链球菌病的防控,我国猪链球菌临床株主要对四环素和红霉素耐药,对青霉素比较敏感[30],这与本试验结果一致。虽然m70分离自流浪猫,但其对抗生素的耐药性仍然十分严重,可见我国抗生素耐药情况不容乐观。
2.5 m70株对小鼠的致病性m70攻毒小鼠7 d后,感染剂量为108 CFU组的小鼠死亡情况严重,死亡率达到60%-80% (3/5-4/5)。3次攻毒试验结果显示,小鼠的平均LD50为5.1×107 CFU(表3)。而用本实验室保存的猪链球菌强毒株HA9801攻毒小鼠7 d后,死亡情况与m70相似,感染剂量为108 CFU组的小鼠死亡情况严重,死亡率达到80%-100% (4/5-5/5),平均LD50为3.9×107 CFU(表3)。由此可见,m70与强毒株HA9801对小鼠的致病力没有显著差异(P<0.05,图4),表明m70株毒力较强。HA9801株是98年江苏爆发猪链球菌疫情时在患有败血症的病猪身上分离到的,毒力较强[31]。m70株与强毒株HA9801 毒力相当,对小鼠模型的致病力较强,在流浪猫身上却无明显症状,这表明猪链球菌虽然可以零星感染其他哺乳动物,但对不同哺乳动物的致病力是有差异的。致病性猪链球菌可能主要导致猪和人发病,但可能对其他哺乳动物致病力较弱,这就提示猪链球菌可能存在从无明显症状的其他哺乳动物传染猪或人,导致猪或人发病的风险。
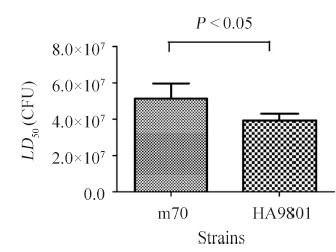 |
| 图 4. 感染不同猪链球菌菌株的小鼠的LD50 Figure 4. LD50 of mice infected with different S. suis strains. |
| 图选项 |
3 结论大部分猪链球菌感染人案例都与职业相关,如屠宰场、养猪场和肉品加工厂等与猪接触的人员[4]。但猪链球菌也会造成多种哺乳动物的偶发感染。本文从流浪猫脏器上分离到猪链球菌9型m70株,在国内尚属首次报道。m70属于新的ST型,16S rRNA系统发育树中处于一个单独的分支,这些特征与我国猪链球菌临床分离株有显著的差别,推测可能与其宿主特异性相关。m70属于我国的流行血清型,且对小鼠致病性较强,提示一些流行血清型的猪链球菌强毒株具有从流浪猫传染人的潜在风险。
参考文献
| [1] | Nga TVT, Nghia HDT, Le Tu TP, Diep TS, Mai NTH, Chau TTH, Sinh DX, Phu NH, Nga TTT, Chau NVV, Campbell J, Hoa NT, Chinh NT, Hien TT, Farrar J, Schultsz C. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagnostic Microbiology and Infectious Disease, 2011, 70(4): 461-467. |
| [2] | Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Veterinary Research Communications, 1997, 21(6): 381-407. |
| [3] | Liu ZJ, Zheng H, Gottschalk M, Bai XM, Lan RT, Ji SB, Liu HC, Xu JG. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis. PloS One, 2013, 8(8): e72070. |
| [4] | Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. The Lancet Infectious Diseases, 2007, 7(3): 201-209. |
| [5] | Hu XS, Zhu FC, Wang H, Chen SY, Wang GH, Sun JZ, Hua CT, Yang HF. Studies on human streptococcal infectious syndrome caused by infected pigs. Chinese Journal of Preventive Medicine, 2000, 34(3): 150-152. (in Chinese)胡晓抒, 朱凤才, 汪华, 陈宋义, 王广和, 孙建中, 华春涛, 杨华富. 人-猪链球菌感染性综合征研究. 中华预防医学杂志, 2000, 34(3): 150-152. |
| [6] | Harel J, Martinez G, Nassar A, Dezfulian H, Labrie SJ, Brousseau R, Moineau S, Gottschalk M. Identification of an inducible bacteriophage in a virulent strain of Streptococcus suis serotype 2. Infection and Immunity, 2003, 71(10): 6104-6108. |
| [7] | Hu P, Yang M, Zhang AD, Wu JY, Chen B, Hua YF, Yu J, Chen HC, Xiao JF, Jin ML. Complete genome sequence of Streptococcus suis serotype 3 strain ST3. Journal of Bacteriology, 2011, 193(13): 3428-3429. |
| [8] | Hu P, Yang M, Zhang AD, Wu JY, Chen B, Hua YF, Yu J, Xiao JF, Jin ML. Complete genome sequence of Streptococcus suis serotype 14 strain JS14. Journal of Bacteriology, 2011, 193(9): 2375-2376. |
| [9] | Huong VTL, Ha N, Huy NT, Horby P, Nghia HDT, Thiem VD, Zhu XT, Hoa NT, Hien TT, Zamora J, Schultsz C, Wertheim HFL, Hirayama K. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerging Infectious Diseases, 2014, 20(7): 1105-1114. |
| [10] | Cruz Colque JI, Devriese LA, Haesebrouck F. Streptococci and enterococci associated with tonsils of cattle. Letters in Applied Microbiology, 1993, 16(2): 72-74. |
| [11] | Devriese LA, Cruz Colque JI, De Herdt P, Haesebrouck F. Identification and composition of the tonsillar and anal enterococcal and streptococcal flora of dogs and cats. Journal of Applied Bacteriology, 1992, 73(5): 421-425. |
| [12] | Devriese LA, Haesebrouck F, de Herdt P, Dom P, Ducatelle R, Desmidt M, Messier S, Higgins R. Streptococcus suis infections in birds. Avian Pathology, 1994, 23(4): 721-724. |
| [13] | Hommez J, Wullepit J, Cassimon P, Castryck F, Ceyssens K, Devriese LA. Streptococcus suis and other streptococcal species as a cause of extramammary infection in ruminants. The Veterinary Record, 1988, 123(24): 626-627. |
| [14] | Sánchez del Rey V, Fernández-Garayzábal JF, Mentaberre G, Briones V, Lavín S, Domínguez L, Gottschalk M, Vela AI. Characterisation of Streptococcus suis isolates from wild boars (Sus scrofa). The Veterinary Journal, 2014, 200(3): 464-467. |
| [15] | Okwumabua O, O'Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiology Letters, 2003, 218(1): 79-84. |
| [16] | Baker GC, Smith JJ, Cowan DA. Review and re-analysis of domain-specific 16S primers. Journal of Microbiological Methods, 2003, 55(3): 541-555. |
| [17] | Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 2007, 24(8): 1596-1599. |
| [18] | 谭世君. 重庆市猪链球菌流行病学调查及GDH基因的克隆和序列分析. 西南大学硕士学位论文, 2007. |
| [19] | King SJ, Leigh JA, Heath PJ, Luque I, Tarradas C, Dowson CG, Whatmore AM. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. Journal of Clinical Microbiology, 2002, 40(10): 3671-3680. |
| [20] | Wilkler MA BK, Cockerill FR, Dudley MN, Eliopoulos GM. Pennsylvania: formance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, 2012. |
| [21] | Chen T, Huang Q, Li ZL, Zhang W, Lu CP, Yao HC. Construction and characterization of a Streptococcus suis serotype 2 recombinant expressing enhanced green fluorescent protein. PLoS One, 2012, 7(7): e39697. |
| [22] | Dekker N, Bouma A, Daemen I, Klinkenberg D, van Leengoed L, Wagenaar JA, Stegeman A. Effect of spatial separation of pigs on spread of Streptococcus suis serotype 9. PLoS One, 2013, 8(4): e61339. |
| [23] | Wisselink HJ, Smith HE, Stockhofe-Zurwieden N, Peperkamp K, Vecht U. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Veterinary Microbiology, 2000, 74(3): 237-248. |
| [24] | Wu ZF, Zhang W, Lu CP. Comparative proteome analysis of secreted proteins of Streptococcus suis serotype 9 isolates from diseased and healthy pigs. Microbial Pathogenesis, 2008, 45(3): 159-166. |
| [25] | Huang JH, Shang KX, Kashif J, Wang LP. Genetic diversity of Streptococcus suis isolated from three pig farms of China obtained by acquiring antibiotic resistance genes. Journal of the Science of Food and Agriculture, 2014, 95(7): 1454-1460. |
| [26] | Ngo TH, Tran TB, Tran TT, Nguyen VD, Campbell J, Pham HA, Huynh HT, Nguyen VV, Bryant JE, Tran TH, Farrar J, Schultsz C. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS One, 2011, 6(3): e17943. |
| [27] | Wang HM, Ke CW, Pan WB, Ke BX, Chen JD, Deng XL, Liu MZ, Chen GR, Yang XF, Zhu ZY. MLST typing of Streptococcus suis isolated from clinical patients in Guangdong Province in 2005. Journal of Southern Medical University, 2008, 28(8): 1438-1441. (in Chinese)王洪敏, 柯昌文, 潘武滨, 柯碧霞, 陈经雕, 邓小玲, 刘美真, 陈国仁, 杨杏芬, 朱振宇. 2005年广东省临床分离猪链球菌的MLST分子分型研究. 南方医科大学学报, 2008, 28(8): 1438-1441. |
| [28] | Xiong ZH, Wei CD, Yang J, Peng JP, Xu XY, Wang Y, Jin Q. Comparative analysis of whole genome structure of Streptococcus suis using whole genome PCR scanning. Science in China. Series C: Life Sciences, 2008, 51(1): 21-26. |
| [29] | Wisselink HJ, Veldman KT, Van den Eede C, Salmon SA, Mevius DJ. Quantitative susceptibility of Streptococcus suis strains isolated from diseased pigs in seven European countries to antimicrobial agents licensed in veterinary medicine. Veterinary Microbiology, 2006, 113(1/2): 73-82. |
| [30] | Chen L, Song YJ, Wei ZG, He HK, Zhang AD, Jin ML. Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. The Journal of Veterinary Medical Science, 2013, 75(5): 583-587. |
| [31] | Yao HC, Chen GQ, Lu CP. Identification of isolates of swine Streptococcus in Jiangsu province during 1998. Journal of Nanjing Agricultural University, 1999, 22(2): 67-70. (in Chinese)姚火春, 陈国强, 陆承平. 猪链球菌1998分离株病原特性鉴定. 南京农业大学学报, 1999, 22(2): 67-70. |
