
 , 李晨曦, 黄兴虎, 周龙, 黄雨欣
, 李晨曦, 黄兴虎, 周龙, 黄雨欣武汉科技大学, 城市建设学院, 武汉 430065
收稿日期: 2020-11-14; 修回日期: 2020-12-10; 录用日期: 2020-12-10
基金项目: 大学生创新创业训练计划项目(No.201910488004);国家自然科学基金(No.51808415)
作者简介: 郭绍东(1987-), 男, 讲师(博士), E-mail: guo_shaodong@126.com
通讯作者(责任作者): 郭绍东
摘要:采用次氯酸钠(NaClO)与亚铁(Fe(Ⅱ))结合处理污泥以提高污泥破解和脱水效能,考察了NaClO和Fe(Ⅱ)投加量的影响,并通过定量和定性分析污泥中胞外聚合物质(EPS)的迁移转化,确定NaClO和Fe(Ⅱ)对污泥脱水和破解的机制.试验结果表明,NaClO和Fe(Ⅱ)投加量(以干泥固体计)分别为44 mg·g-1和66 mg·g-1时,污泥毛细吸吮时间(CST)从原泥的112 s下降到66.3 s.同时,NaClO/Fe(Ⅱ)处理污泥能够使上清液中化学需氧量(COD)显著升高,并使污泥中紧密束缚型EPS(TB-EPS)和松散束缚型EPS(LB-EPS)转化成溶解性EPS(S-EPS),导致S-EPS的溶解性有机碳(DOC)含量升高,而LB-EPS和TB-EPS的DOC含量降低.此外,NaClO/Fe(Ⅱ)还能使污泥中有机物质矿化,使3种EPS中蛋白质类和腐殖酸类物质减少.NaClO和Fe(Ⅱ)能够改善污泥脱水性能,强化污泥破解效果,利于污泥后续的处理处置.
关键词:次氯酸钠(NaClO)二价铁污泥脱水氧化胞外聚合物质
Effects of sodium hypochlorite and ferrous iron on sludge disintegration and dewatering
GUO Shaodong

 , LI Chenxi, HUANG Xinghu, ZHOU Long, HUANG Yuxin
, LI Chenxi, HUANG Xinghu, ZHOU Long, HUANG YuxinSchool of Urban Construction, Wuhan University of Science and Technology, Wuhan 430065
Received 14 November 2020; received in revised from 10 December 2020; accepted 10 December 2020
Abstract: The effects of NaClO and Fe(Ⅱ) dosages on sludge disintegration and dewatering properties were investigated, and the underlying mechanism of this NaClO/Fe(Ⅱ) conditioning was explored by analyzing the transformation of sludge extracellular polymeric substance (EPS). results showed that when NaClO and Fe(Ⅱ) dosage were 44 mg·g-1 and 66 mg·g-1 drying solid, respectively, the capillary sucking time (CST) decreased from 112 s to 66.3 s for raw sludge. Moreover, chemical oxygen demand (COD) significantly increased in conditioned sludge supernatant, and tightly bound EPS (TB-EPS) and loosely bound EPS (LB-EPS) were transformed into soluble EPS (S-EPS) under NaClO/Fe(Ⅱ) conditioning. As a result, dissolved organic carbon (DOC) of S-EPS increased while DOC of TB-EPS and LB-EPS decreased. In addition, the organic substances in sludge were mineralized by NaClO/Fe(Ⅱ), resulting in the reduction of the protein and humic substances in the three EPSs. These results suggest that NaClO/Fe(Ⅱ) could improve sludge dewaterability and disintegration efficiency, which is benefit to the subsequent sludge treatment and disposal.
Keywords: NaClOferroussludge dewateringoxidationextracellular polymeric substance
1 引言(Introduction)污水处理厂剩余污泥产量大、含水率高, 存在致病微生物、重金属等污染, 同时又富含可供植物利用的营养物质, 若不科学处理处置会引起环境二次污染及资源浪费, 因此, 人们越来越重视剩余污泥的处理处置, 对污泥进行无害化、减量化、资源化处理.其中, 污泥无害化主要是处理致病微生物、重金属等的污染(Wu et al., 2020), 减量化主要是解决污泥中水分含量大的问题.对于市政污水处理厂污泥, 减量化程度最高的处理手段是污泥脱水(Wong et al., 2016).然而, 由于污泥中微生物群落具有高度亲水性的胶态结构, 因此很难直接脱水(Christensen et al., 2015; Zhang et al., 2016).为此有很多研究开始着力于考察污泥脱水的影响因素及适用的调理方法(Ge et al., 2020; Li et al., 2020; Liang et al., 2020b; Liu et al., 2020; Wang et al., 2020).胞外聚合物质(EPS)作为污泥絮体中的主要有机组分, 占比达到50%以上.研究表明, EPS的物化性质会影响到污泥絮体的亲疏水性, 且EPS是导致污泥中结合水存在的主要原因, 因而被认为是影响污泥脱水效果的决定性因素(Xiao et al., 2017; Xu et al., 2018; Cai et al., 2019).假如使用一定的技术方法能使EPS大量溶解将有助于释放污泥中的结合水和间隙水, 并改善脱水效能.其中, 以高级氧化法为代表的旨在破坏细胞壁和EPS改善污泥脱水性能的方法被广泛报道(Li et al., 2019; Wei et al., 2019; Ge et al., 2020).如Li等(2019)使用高铁酸钾和超声强化污泥脱水和破解效能时, 通过检测溶解性EPS、总有机碳等发现二者结合对污泥破解十分有效;Wei等(2019)先使用H2O2、NaClO和KMnO4预氧化污泥, 然后再使用FeCl3等絮凝剂调理, 可以显著改善污泥脱水性能和污泥比阻(SRF);Ge等(2020)研究发现使用活化过硫酸盐和单宁酸调理可使污泥的毛细吸吮时间(CST)等显著降低.
次氯酸钠(NaClO)作为一种常用的氧化剂, 在污水处理领域有着较多的研究.如Wei等(2019)研究发现, 单独的次氯酸钠在污泥固有的pH下对污泥脱水性能具有恶化作用, 因此不能单独作为污泥调理剂.而在一定条件下, 次氯酸盐溶液中投入亚铁离子会发生类芬顿反应(式(1))(Murrieta et al., 2020), 产生强氧化性的羟基自由基(·OH), 可用于破坏EPS结构, 同时生成的Fe(Ⅲ)也有利于污泥絮凝(Xu et al., 2016; Xiao et al., 2018; Tao et al., 2019; Liang et al., 2020a).如Yu等(2019)研究发现, 次氯酸钙和亚铁离子的投加量分别为106.1 mg·g-1和234.5 mg·g-1(以挥发性固体计)时, 毛细吸吮时间(CST)从172.7 s下降到29.3 s, 污泥深度脱水效果得到显著改善.
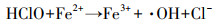 | (1) |
2 材料和方法(Materials and methods)2.1 原泥污泥取自武汉市某一采用改良氧化沟工艺的污水处理厂(日处理量5万t), 为二沉池到厌氧池的回流污泥.收集的污泥用30目筛子除去大颗粒物质, 浓缩至含水率大约为95.2%.使用前将污泥置于塑料桶中并在4 ℃下储存, 污泥样品相关指标均在3 d内检测完成.试验用污泥pH约为7.4, 悬浮固体(SS)浓度约为47.6 g·L-1, 挥发性悬浮固体(VSS)浓度约为21.9 g·L-1.
2.2 试验及检测方法试验过程中, 次氯酸钠(NaClO)投加量分别为11、22、44 mg·g-1(以干泥固体计, 下同), 硫酸亚铁(以Fe2+计)投加量分别为22、44、66 mg·g-1(以干泥固体计, 下同).试验步骤如下:在每个烧杯中倒入400 g含水率为95.3%的浓缩后污泥, 投入22(或44、66) mg·g-1硫酸亚铁粉末, 在200 r·min-1转速下搅拌2 min;然后分别投入11、22、44 mg·g-1的NaClO, 在150 r·min-1转速下搅拌15 min;提取污泥进行后续检测.
污泥上清液化学需氧量(COD)采用重铬酸钾法检测;污泥胞外聚合物质(EPS)采用热提取法提取(Guo et al., 2019), 分为溶解性EPS(S-EPS)、松散附着型EPS(LB-EPS)和紧密附着型EPS(TB-EPS), 检测各EPS的溶解性有机碳(DOC)含量.在进行DOC检测时, 各样品先用0.45 μm醋酸纤维微滤膜过滤.
2.3 主要试剂及检测仪器主要检测仪器有CST检测仪(Tritonel 304M型, 英国)、三维荧光激发-发射光谱仪(3D-EEM, FL-7000, 日立公司, 日本)、总有机碳分析仪(multi N/C 2100, 耶拿, 德国)和Zeta sizer nano S90(马尔文, 英国). FeSO4·7H2O和NaClO均为分析纯, 购自天津市巴斯夫化工有限公司.
3 结果与讨论(Results and discussion)3.1 NaClO和Fe(Ⅱ)对污泥脱水性能的影响经过NaClO和Fe(Ⅱ)调理之后, 污泥CST变化如图 1所示.由图可见, 投加NaClO和Fe(Ⅱ)后污泥CST有所下降, 当Fe(Ⅱ)投加量大于22 mg·g-1时, CST下降趋势较明显.特别地, 当NaClO和Fe(Ⅱ)投加量分别为44和66 mg·g-1时, CST从原泥的112 s下降到66.3 s, 下降幅度达到40.8%.结果表明, 经NaClO和Fe(Ⅱ)调理后污泥脱水性能得到较显著的改善.研究发现, 单独投加NaClO调理污泥会恶化污泥脱水性能(Wei et al., 2019), 使污泥的CST升高;而如果单独投加Fe(Ⅱ)则不能被氧化成Fe(Ⅲ), 对污泥的絮凝作用很有限, 也不能改善污泥脱水性能.而NaClO与Fe(Ⅱ)相结合后, 污泥脱水性能得到了较显著的改善, 可见二者的相互作用在一定程度上改变了泥水结合特性.
图 1(Fig. 1)
 |
| 图 1 NaClO/Fe(Ⅱ)作用下污泥CST的变化 Fig. 1Change of sludge CST under NaClO/Fe (Ⅱ) conditioning |
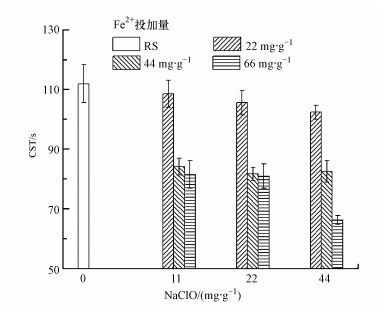
3.2 NaClO和Fe(Ⅱ)破解污泥的效果污泥上清液中CODCr变化如图 2所示, CODCr随着NaClO投加量的增加而升高, 特别是投加44 mg·g-1 NaClO时, 相校原始污泥, CODCr增加4倍以上.研究表明, NaClO与污泥作用时, 会氧化污泥上清液中的一部分有机物质, 然后再破坏污泥结构, 释放有机物质, 这时污泥上清液中的有机质增加较少;而当NaClO投加量达到一定时, 氧化剂量足够氧化污泥絮体, 甚至细胞, 释放有机物质, 使得CODCr升高较明显(Wei et al., 2019).Fe(Ⅱ)在一定程度上促进了NaClO氧化污泥絮体, 释放CODCr, 表明NaClO和Fe(Ⅱ)对污泥破解产生了协同作用.
图 2(Fig. 2)
 |
| 图 2 NaClO/Fe(Ⅱ)作用下污泥上清液中CODCr的变化 Fig. 2Change of CODCr in sludge supernate under NaClO/Fe (Ⅱ) conditioning |
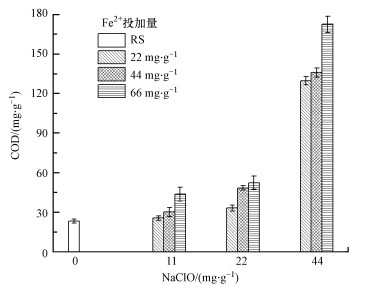
污泥中提取的S-EPS、LB-EPS和TB-EPS的DOC含量变化如图 3所示.对S-EPS的DOC而言, 当保持NaClO投加量不变, DOC含量随着Fe(Ⅱ)投加量增加而有所降低, 特别是当Fe(Ⅱ)投加量从22 mg·g-1增加到44 mg·g-1时, DOC下降明显, 此后基本保持不变;而当保持Fe(Ⅱ)投加量不变时, DOC含量随着NaClO投加量的增加有所下降.对于LB-EPS和TB-EPS的DOC, 当NaClO投加量不变时, Fe2+投加量升高, DOC含量下降;并且当Fe2+投加量不变, NaClO投加量升高时, DOC含量也呈下降趋势.当NaClO和Fe(Ⅱ)的投加量分别为44 mg·g-1和66 mg·g-1时, TB-EPS的DOC从原泥的26.9 mg·g-1下降到11.2 mg·g-1.试验结果表明, NaClO既能氧化污泥上清液中的有机质, 同时又能破坏污泥的EPS, 对减少污泥中的有机质起到了显著的作用.这主要得益于Fe(Ⅱ)和NaClO产生了类芬顿反应(Murrieta et al., 2020).
图 3(Fig. 3)
 |
| 图 3 NaClO/Fe(Ⅱ)作用下污泥中S-EPS (a)、LB-EPS (b) 及TB-EPS(c)的DOC变化 Fig. 3Changes of DOC in sludge S-EPS (a), LB-EPS (b) and TB-EPS (c) under NaClO/Fe (Ⅱ) conditioning |
污泥S-EPS的三维荧光激发-发射(3D-EEM)图谱如图 4所示, 存在的主要荧光特征峰有芳香族类蛋白质T1(λEX/λEM=230 nm/308 nm)(Zhu et al., 2015)、可溶解性微生物副产物T2(λEX/λEM=270~280 nm/308 nm)(Bourven et al., 2012)、色氨酸-蛋白质类物质T3(λEX/λEM=280~290 nm/320~330 nm)(Pang et al., 2014)、腐殖酸类物质(λEX/λEM= 325~350 nm/430~446 nm;λEX/λEM=275 nm/350 nm)(Wang et al., 2009).
图 4(Fig. 4)
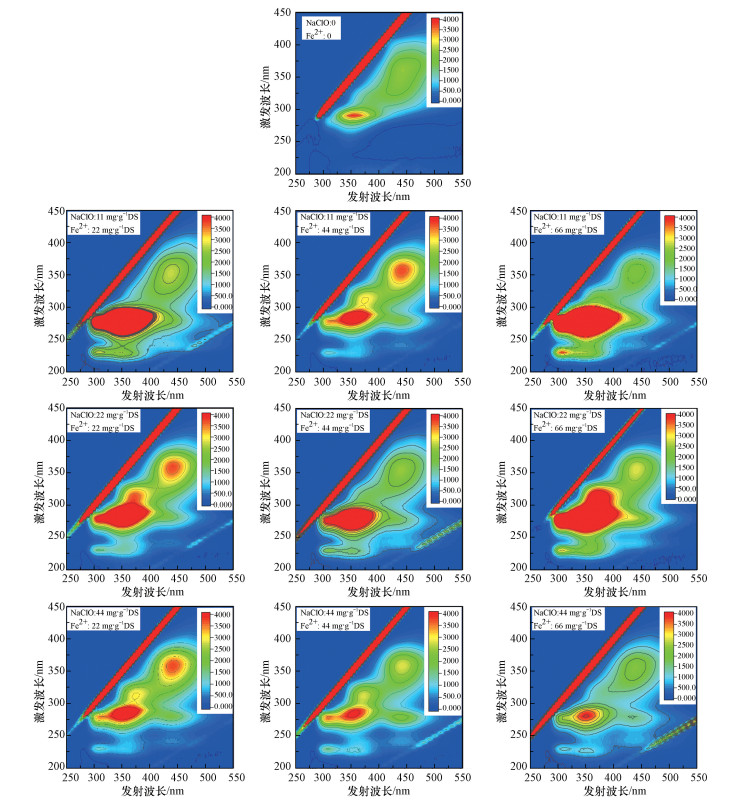 |
| 图 4 NaClO/Fe(Ⅱ)作用下污泥S-EPS的三维荧光图谱 Fig. 43D-EEM fluorescence spectra of S-EPS under NaClO/Fe (Ⅱ) conditioning |
原泥上清液中荧光类物质峰强度较低, 单独考察NaClO的作用时, 保持Fe(Ⅱ)投加量不变, 当投加11 mg·g-1 NaClO后溶液中的类蛋白质、腐殖酸类荧光物质峰强度明显增强;随着NaClO投加量的增加, 上述荧光峰强度有所降低, 特别是当次氯酸钠投加量为44 mg·g-1时, 荧光强度最弱.当NaClO投加量不变, 各荧光峰强度随着Fe(Ⅱ)投加量的增加呈现变弱的趋势.结果表明, NaClO和Fe(Ⅱ)结合处理污泥后, 使得溶解性的蛋白质类和腐殖酸类物质含量降低.
LB-EPS的3D-EEM图谱如图 5所示, LB-EPS中主要存在的荧光类物质有芳香族类蛋白质、可溶解性微生物副产物、色氨酸-蛋白质类物质、腐殖酸类物质.相对而言, 原泥的LB-EPS中可溶解性微生物副产物和腐殖酸类物质较多, 而色氨酸-蛋白质类物质和芳香族类蛋白质较少.投加NaClO和Fe(Ⅱ)处理污泥之后, 相对原泥LB-EPS而言, 溶解性微生物副产物、芳香族类蛋白质和腐殖酸类物质显著增加.当NaClO投加量达到22 mg·g-1及以上时, 随着Fe(Ⅱ)投加量的增加, 腐殖酸类物质明显减少.而当Fe(Ⅱ)投加量不变时, 随着NaClO投加量的增加, 色氨酸-蛋白质类物质、可溶解性微生物副产物和腐殖酸类物质明显减少, 芳香族类蛋白质也呈减少趋势.
图 5(Fig. 5)
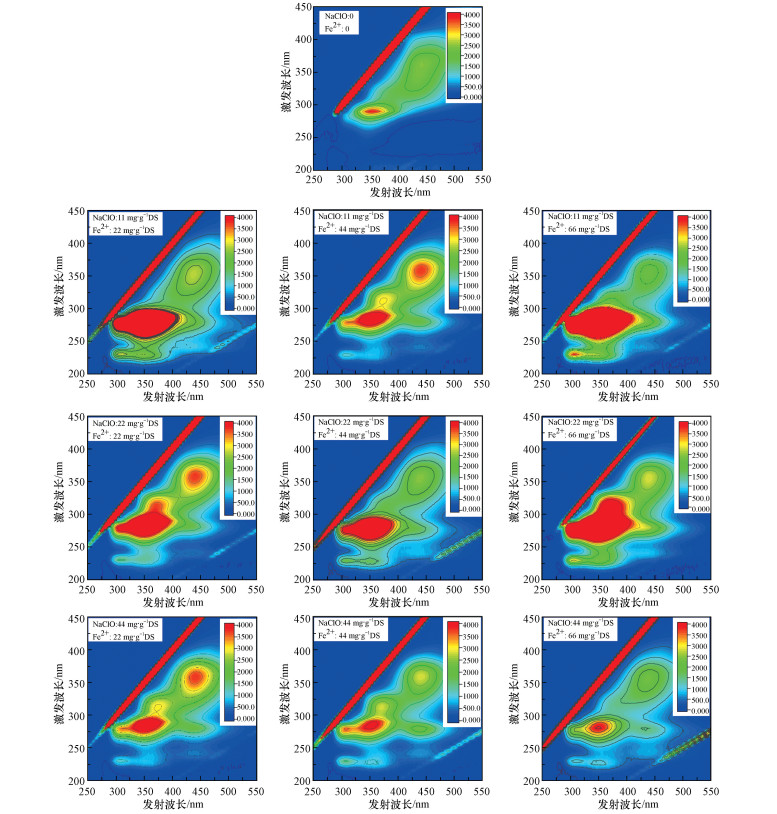 |
| 图 5 NaClO/Fe(Ⅱ)作用下污泥LB-EPS三维荧光图谱 Fig. 53D-EEM fluorescence spectra of LB-EPS under NaClO/Fe (Ⅱ) conditioning |
不同NaClO和Fe(Ⅱ)投加量下TB-EPS的3D-EEM图谱如图 6所示, 存在的主要荧光类物质有芳香族类蛋白质、可溶解性微生物副产物、色氨酸-蛋白质类物质、腐殖酸类物质.相对而言, 原泥TB-EPS中色氨酸-蛋白质类物质、可溶解性微生物副产物较多, 而腐殖酸类物质和芳香族类蛋白质较少.当NaClO和Fe(Ⅱ)投加量分别为11 mg·g-1和22 mg·g-1时, 可溶解性微生物副产物较多, 芳香族类蛋白质和腐殖酸类物质有所增加.当NaClO投加量不变时, 随着Fe(Ⅱ)投加量的增加, 腐殖酸类物质明显减少, 而香族类蛋白质较显著增加.而当Fe(Ⅱ)投加量不变时, 随着NaClO投加量的增加, 色氨酸-蛋白质类物质、可溶解性微生物副产物和腐殖酸类物质明显减少.TB-EPS中含有的物质与LB-EPS中基本相同, 其变化规律和趋势也大体相似.可见较高投加量的NaClO和Fe(Ⅱ)能够减少污泥中腐殖酸类物质.
图 6(Fig. 6)
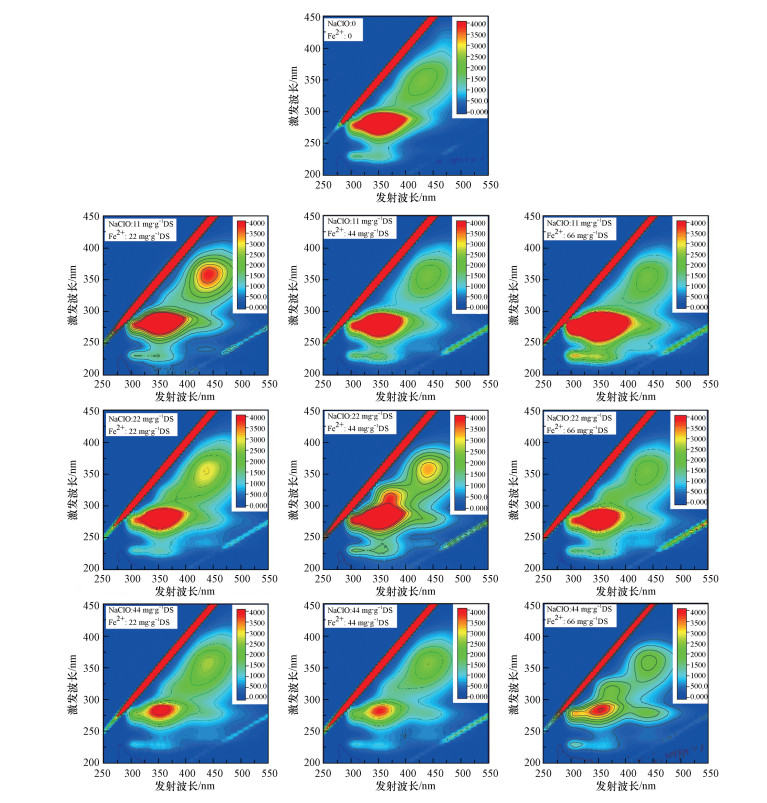 |
| 图 6 NaClO/Fe(Ⅱ)作用下污泥TB-EPS三维荧光图谱 Fig. 63-D EEM fluorescence spectra of TB-EPS under NaClO/Fe(Ⅱ) conditioning |
对污泥提取的不同层的EPS进行分析, 发现一定量的NaClO/Fe(Ⅱ)处理可以减少污泥中EPS中的荧光类物质, 主要包括色氨酸-蛋白质类和腐殖酸类物质.通过上述结果分析可知, 在不同的NaClO/Fe(Ⅱ)投加量下, 荧光类物质发生了不同的迁移转化过程, 当投加量不足时, NaClO/Fe(Ⅱ)先氧化溶解性的荧光类物质, 随着药剂投加量增加, 开始氧化LB-EPS和TB-EPS中的物质.结合上清液的DOC浓度分析认为, NaClO/Fe(Ⅱ)对EPS中的荧光类物质起到了矿化作用.
不同NaClO和Fe(Ⅱ)投加量下污泥Zeta电位变化如图 7所示, 投加NaClO和Fe(Ⅱ)下污泥Zeta电位有所升高, NaClO投加量较低时, Zeta电位随着Fe(Ⅱ)投加量的升高而升高.当Fe(Ⅱ)投加量不变时, Zeta电位也随着NaClO投加量的增加而升高.特别地, 当NaClO和Fe(Ⅱ)投加量分别44 mg·g-1和66 mg·g-1时, 污泥Zeta电位升高最明显, 达到-8 mV左右.虽然NaClO会破坏污泥结构和蛋白质, 甚至使细胞破解, 释放亲水性物质, 导致Zeta电位降低, 然而由于Fe(Ⅱ)的投加, 在一定程度上中和负电性, 因而在试验的投加量下使负电性减弱.
图 7(Fig. 7)
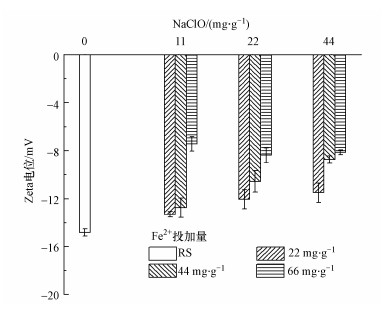 |
| 图 7 NaClO/Fe(Ⅱ)作用下污泥Zeta电位变化 Fig. 7Change of zeta potential under NaClO/Fe (Ⅱ) conditioning |
3.3 机制分析污泥脱水和破解效果与污泥EPS中的物质迁移转化有着密切关系(毕薇薇等, 2020;石琦等, 2020).利用NaClO/Fe(Ⅱ)调理污泥时, 污泥上清液中DOC含量有所增加, TB-EPS和LB-EPS中DOC含量减少, 主要得益于NaClO/Fe(Ⅱ)的氧化作用, 污泥中有机物质发生迁移转化, 利于絮体解体及释放絮体中的束缚水.同时, 污泥S-EPS、LB-EPS和TB-EPS中蛋白质和腐殖酸类物质都减少且部分被矿化, 而污泥中的蛋白质类物质越少越不易于锁住污泥中的束缚水(Yu et al., 2008), 特别是色氨酸-蛋白质类物质的减少有利于促进污泥脱水(Zhen et al., 2012; Baudez et al., 2013; Zhen et al., 2013; Sik et al., 2016).此外, 污泥Zeta电位的负电性减弱, 可以促进污泥聚集成大颗粒, 从而利于脱水(Wu et al., 2020).因此, NaClO/Fe(Ⅱ)可以显著提高污泥脱水性能.可见NaClO/Fe(Ⅱ)调理污泥时, NaClO不仅能够破坏污泥絮体和EPS使污泥解体(Wei et al., 2019), 同时可以氧化亚铁为三价铁, 起到原位混凝作用.
4 结论(Conclusions)1) NaClO/Fe(Ⅱ)协同产生的氧化-絮凝作用降低了污泥的电负性, 减少了TB-EPS含量, 并显著改善污泥脱水性能, 当NaClO和Fe(Ⅱ)投加量分别为44 mg·g-1和66 mg·g-1时, 污泥CST显著降低, 从原泥的112 s下降到66.3 s.
2) NaClO/Fe(Ⅱ)能够氧化污泥絮体, 使得TB-EPS和LB-EPS转化成LB-EPS, 同时减少污泥中色氨酸-蛋白质类和腐殖酸类物质的含量.
3) NaClO/Fe(Ⅱ)的强氧化性能够矿化污泥中的有机物质, 使LB-EPS和TB-EPS中DOC含量降低. 当NaClO和Fe(Ⅱ)投加量分别为44 mg·g-1和66 mg·g-1时, LB-EPS的DOC从原泥的31.8 mg·g-1下降到13.1 mg·g-1, TB-EPS的DOC从原泥的26.9 mg·g-1下降到11.2 mg·g-1.
参考文献
| Baudez J C, Gupta R K, Eshtiaghi N, et al. 2013. The viscoelastic behaviour of raw and anaerobic digested sludge: Strong similarities with soft-glassy materials[J]. Water Research, 47(1): 173-180. DOI:10.1016/j.watres.2012.09.048 |
| 毕薇薇, 阮书瑜, 陈吴傲啸, 等. 2020. 二价铁活化过氧化钙提高剩余活性污泥的脱水性能[J]. 环境科学, 41(12): 5544-5551. |
| Bourven I, Costa G, Guibaud G. 2012. Qualitative characterization of the protein fraction of exopolymeric substances (EPS) extracted with EDTA from sludge[J]. Bioresource Technology, 104: 486-496. DOI:10.1016/j.biortech.2011.11.033 |
| Cai M, Wang Q, Wells G, et al. 2019. Improving dewaterability and filterability of waste activated sludge by electrochemical Fenton pretreatment[J]. Chemical Engineering Journal, 362: 525-536. DOI:10.1016/j.cej.2019.01.047 |
| Christensen M L, Keiding K, Nielsen P H, et al. 2015. Dewatering in biological wastewater treatment: A review[J]. Water Research, 82: 14-24. DOI:10.1016/j.watres.2015.04.019 |
| Ge D, Dong Y, Zhang W, et al. 2020. A novel Fe2+/persulfate/tannic acid process with strengthened efficacy on enhancing waste activated sludge dewaterability and mechanism insight[J]. Science of the Total Environment, 733: 139146. DOI:10.1016/j.scitotenv.2020.139146 |
| Guo S, Liang H, Bai L, et al. 2019. Synergistic effects of wheat straw powder and persulfate/Fe(Ⅱ) on enhancing sludge dewaterability[J]. Chemosphere, 215: 333-341. DOI:10.1016/j.chemosphere.2018.10.008 |
| Li W, Yu N, Fang A, et al. 2019. Co-treatment of potassium ferrate and ultrasonication enhances degradability and dewaterability of waste activated sludge[J]. Chemical Engineering Journal, 361: 148-155. DOI:10.1016/j.cej.2018.12.058 |
| Li Y, Wang D, Xu Q, et al. 2020. New insight into modification of extracellular polymeric substances extracted from waste activated sludge by homogeneous Fe(Ⅱ)/persulfate process[J]. Chemosphere, 247: 125804. DOI:10.1016/j.chemosphere.2019.125804 |
| Liang J, Huang J, Zhang L, et al. 2020a. High-level waste activated sludge dewaterability using Fenton-like process based on pretreated zero valent scrap iron as an in-situ cycle iron donator[J]. Journal of Hazardous Materials, 391: 122219. DOI:10.1016/j.jhazmat.2020.122219 |
| Liang J, Zhang L, Ye M, et al. 2020b. Evaluation of the dewaterability, heavy metal toxicity and phytotoxicity of sewage sludge in different advanced oxidation processes[J]. Journal of Cleaner Production, 265: 121839. DOI:10.1016/j.jclepro.2020.121839 |
| Liu C, Chen X E, Cai H. 2020. Aerobically digested sludge conditioning by Fe2+/citrate chelated-Fe2+ activated peroxymonosulfate oxidation[J]. Chemical Engineering Journal, 400: 125954. DOI:10.1016/j.cej.2020.125954 |
| Murrieta M F, Brillas E, Nava J L, et al. 2020. Photo-assisted electrochemical production of HClO and Fe2+ as Fenton-like reagents in chloride media for sulfamethoxazole degradation[J]. Separation and Purification Technology, 250: 117236. DOI:10.1016/j.seppur.2020.117236 |
| Pang L N, Ni J R, Tang X Y. 2014. Fast characterization of soluble organic intermediates and integrity of microbial cells in the process of alkaline anaerobic fermentation of waste activated sludge[J]. Biochemical Engineering Journal, 86: 49-56. DOI:10.1016/j.bej.2014.03.005 |
| 石琦, 黄润垚, 王洪涛, 等. 2020. 酸化/氧化/絮凝联合调理污泥的全过程研究[J]. 环境污染与防治, 42(10): 1263-1268+1284. |
| Sik K M, Ki-Myeong L, Hyung-Eun K, et al. 2016. Disintegration of waste activated sludge by thermally-activated persulfates for enhanced dewaterability[J]. Environmental Science & Technology, 50(13): 7015-7155. |
| Tao S, Yang J, Hou H, et al. 2019. Enhanced sludge dewatering via homogeneous and heterogeneous Fenton reactions initiated by Fe-rich biochar derived from sludge[J]. Chemical Engineering Journal, 372: 966-977. DOI:10.1016/j.cej.2019.05.002 |
| Wang H, Hu H, Wang H J, et al. 2020. Comprehensive investigation of the relationship between organic content and waste activated sludge dewaterability[J]. Journal of Hazardous Materials, 394: 122547. DOI:10.1016/j.jhazmat.2020.122547 |
| Wang Z W, Wu Z C, Tang S J. 2009. Extracellular polymeric substances (EPS) properties and their effects on membrane fouling in a submerged membrane bioreactor[J]. Water Research, 43: 2504-2512. DOI:10.1016/j.watres.2009.02.026 |
| Wei H, Tang Y, Shoeib T, et al. 2019. Evaluating the effects of the preoxidation of H2O2, NaClO, and KMnO4 and reflocculation on the dewaterability of sewage sludge[J]. Chemosphere, 234: 942-952. DOI:10.1016/j.chemosphere.2019.06.131 |
| Wong J W C, Murugesan K, Selvam A, et al. 2016. Dewatering of saline sewage sludge using iron-oxidizing bacteria: Effect of substrate concentration[J]. Bioresource Technology, 213: 31-38. DOI:10.1016/j.biortech.2016.03.118 |
| Wu B R, Dai X H, Chai X L. 2020. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations[J]. Water Research, 180: 18. |
| Xiao K, Pei K, Wang H, et al. 2018. Citric acid assisted Fenton-like process for enhanced dewaterability of waste activated sludge with in-situ generation of hydrogen peroxide[J]. Water Research, 140: 232-242. DOI:10.1016/j.watres.2018.04.051 |
| Xiao K, Seow W Y, Chen Y, et al. 2017. Effects of thermal-Fe (Ⅱ) activated oxone treatment on sludge dewaterability[J]. Chemical Engineering Journal, 322: 463-471. DOI:10.1016/j.cej.2017.04.055 |
| Xu H, Shen K, Ding T, et al. 2016. Dewatering of drinking water treatment sludge using the Fenton-like process induced by electro-osmosis[J]. Chemical Engineering Journal, 293: 207-215. DOI:10.1016/j.cej.2016.02.025 |
| Xu Q, Wang Q, Zhang W, et al. 2018. Highly effective enhancement of waste activated sludge dewaterability by altering proteins properties using methanol solution coupled with inorganic coagulants[J]. Water Research, 138: 181-191. |
| Yu G H, He P J, Shao L M, et al. 2008. Stratification structure of sludge flocs with implications to dewaterability[J]. Environmental Science & Technology, 42: 7944-7949. |
| Yu W, Wen Q, Yang J, et al. 2019. Unraveling oxidation behaviors for intracellular and extracellular from different oxidants (HOCl vs.H2O2) catalyzed by ferrous iron in waste activated sludge dewatering[J]. Water Research, 148: 60-69. |
| Zhang J, Zhang J, Tian Y, et al. 2016. Changes of physicochemical properties of sewage sludge during ozonation treatment: Correlation to sludge dewaterability[J]. Chemical Engineering Journal, 301: 238-248. DOI:10.1016/j.cej.2016.04.151 |
| Zhen G, Lu X, LI Y, et al. 2012. Novel insights into enhanced dewaterability of waste activated sludge by Fe(Ⅱ)-activated persulfate oxidation[J]. Bioresource Technology, 119: 7-14. |
| Zhen G Y, Lu X Q, Li Y Y, et al. 2013. Innovative combination of electrolysis and Fe(Ⅱ)-activated persulfate oxidation for improving the dewaterability of waste activated sludge[J]. Bioresource Technology, 136: 654-663. |
| Zhu L, Zhou J H, Lv M L, et al. 2015. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE[J]. Chemosphere, 121: 26-32. |
