2.宾夕法尼亚印第安纳大学化学系,印第安纳郡 15705
2.Department of Chemistry, Indiana University of Pennsylvania, Pennsylvania 15705, USA
高浓度氨氮及大量腐殖质的存在一直以来都是膜电容去离子技术(membrane capacitive deionization,MCDI)应用于垃圾渗滤液除盐的重要难题。采用PE离子交换膜作为膜材料,以活性交联碳布(activated carbon cloth,ACC)作为吸附材料组装MCDI装置,并对含有不同浓度梯度的氨氮和腐殖酸的模拟垃圾渗滤液进行吸附除盐实验。结果表明,垃圾渗滤液中氨氮浓度的增加会使MCDI对氨氮的去除量上升,但会导致Na
)浓度会导致所有无机离子特别是阴离子吸附量的下降,且腐殖酸的存在也会降低MCDI对所有离子的再吸附效果,减小MCDI的使用寿命。对垃圾渗滤液进行预处理,将氨氮和腐殖酸的浓度降低,有利于提高MCDI的去离子效果。
High concentration ammonia-nitrogen and humic acid are main challenges to apply membrane capacitive deionization (MCDI) to desalinate the landfill leachate plumes. In this study, the activated carbon cloth (ACC) electrodes coated with polyethylene ion exchange membranes were used in a MCDI system. The desalination performance of the MCDI was studied on the treatment of synthetic landfill leachate plumes containing different concentration gradients of ammonia nitrogen and humic acid. The results showed that the increase of ammonia nitrogen concentration in landfill leachate led to an increase of ammonia nitrogen removal by MCDI, while the adsorption amount of Na
removals were less affected. The presence of low concentration humic acid (less than 1 500 mg·L
by MCDI to some extent. However, a high humic acid concentrations (greater than 1 500 mg·L
) could reduce the adsorption amount of all inorganic ions, especially for anions. The humic acid presence also reduced the re-adsorption effect of all ions by MCDI, and shortened the service life of MCDI accordingly. Pretreatment of landfill leachate could reduce the concentration of ammonia nitrogen and humic acid, which was beneficial for the improvement of the deionization efficiency of MCDI.
.
MCDI对5种模拟垃圾渗滤液中阳离子的吸附量
Adsorption amount of cations in five artificial landfill leachates by MCDI
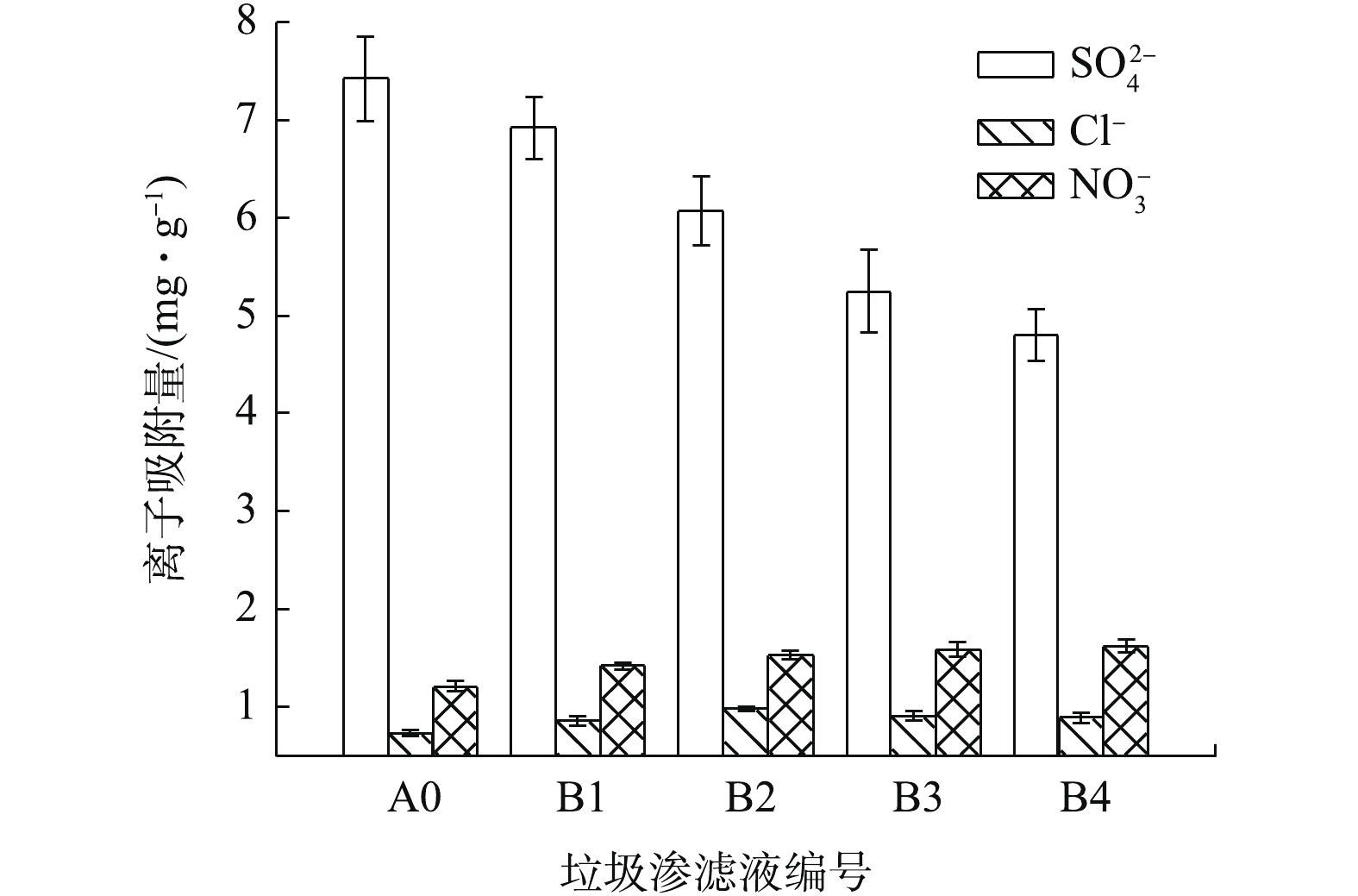
MCDI对5种模拟垃圾渗滤液中阴离子的吸附量
Adsorption amount of anions in five artificial landfill leachates by MCDI
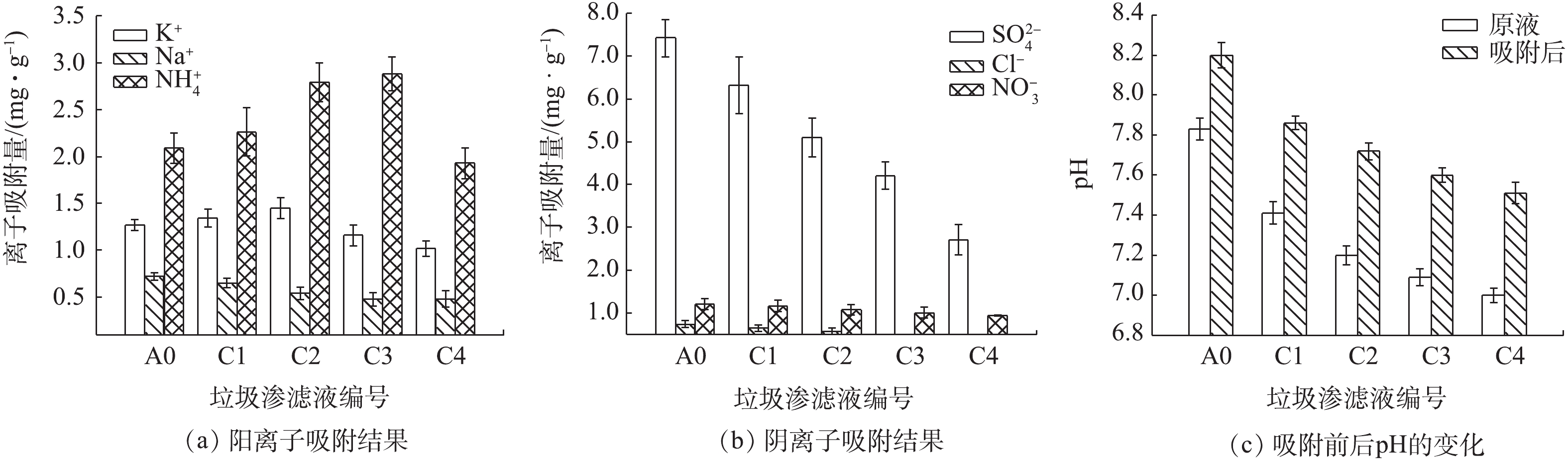
MCDI对模拟垃圾渗滤液A0、C1、C2、C3和C4的吸附结果
Adsorption effects of artificial landfill leachates of A0, C1, C2, C3 and C4 by MCDI
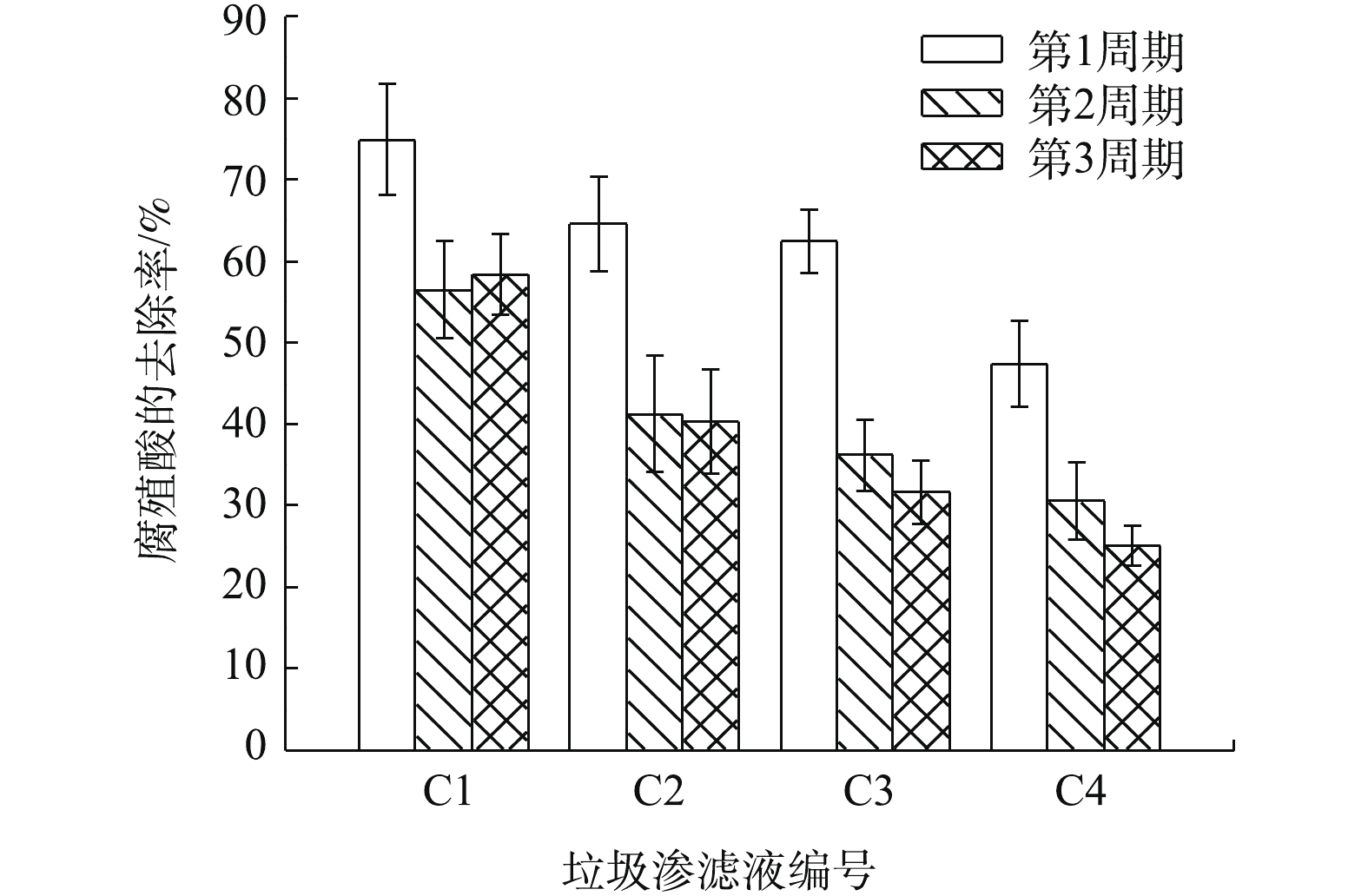
3个吸附周期下模拟垃圾渗滤液C1、C2、C3和C4的腐殖酸去除率
Humic acid removal rate in landfill leachates of C1、C2、C3 and C4 in three adsorption cycles
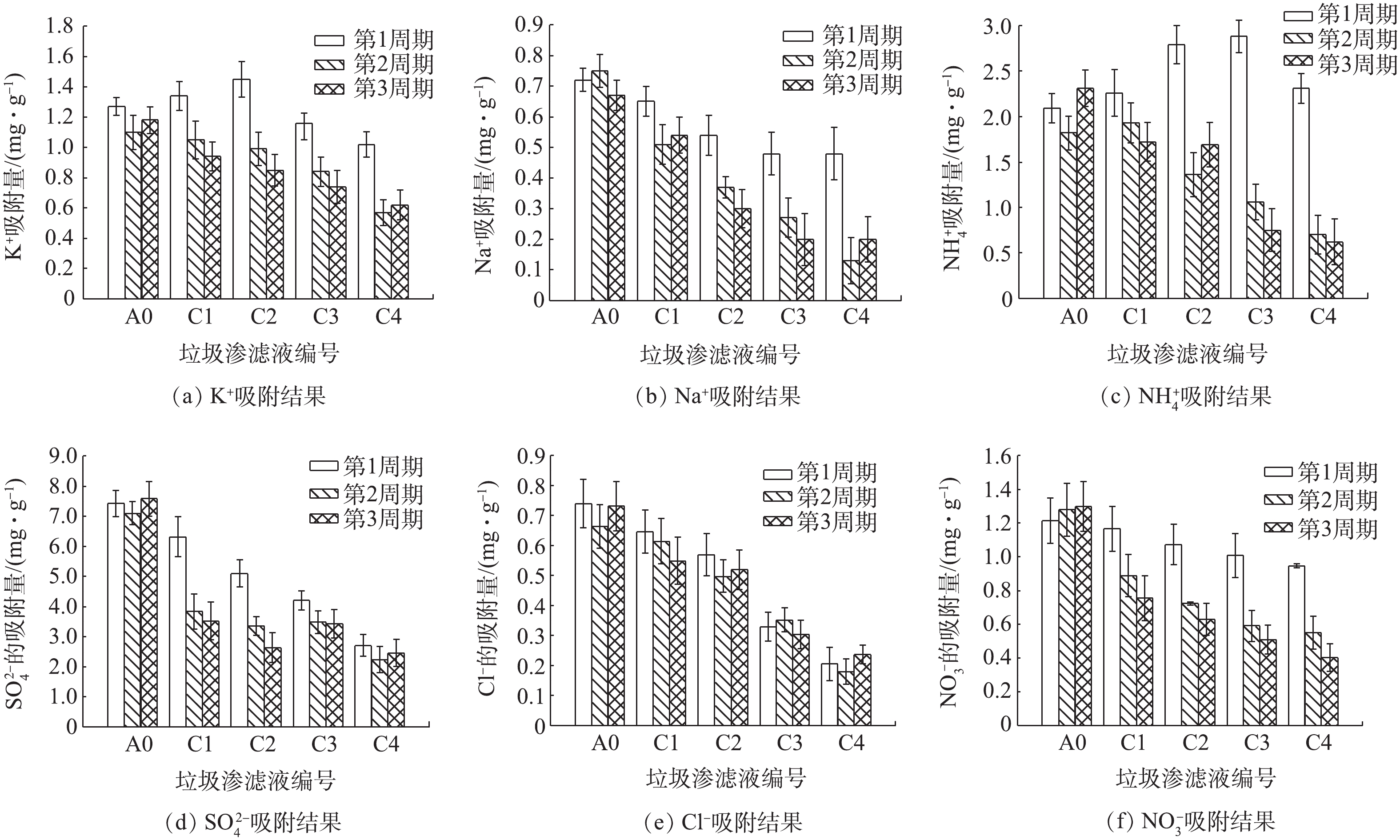
3个吸附周期下模拟垃圾渗滤液A0、C1、C2、C3、C4的吸附结果
Adsorption results of landfill leachates of A0, C1, C2, C3, C4 in three adsorption cycles
| [1] | RENOU S, POULAIN S, GIVAUDAN J, et al. Treatment process adapted to stabilized leachates: Lime precipitation-prefiltration-reverse osmosis[J]. Journal of Membrane Science, 2008, 313(1/2): 9-22. |
| [2] | KI-HOON KANG, H S, HEEKYUNG P. Characterization of humic substances present in landfill leachates with different landfill ages and its implications[J]. Water Research, 2002, 36(16): 4023-4032. doi: 10.1016/S0043-1354(02)00114-8 |
| [3] | HE P, XUE J, SHAO L, et al. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill[J]. Water Research, 2006, 40(7): 1465-1473. doi: 10.1016/j.watres.2006.01.048 |
| [4] | THOMAS H, CHRISTENSEN P, POUL L, et al. Biogeochemistry of landfill leachate plumes[J]. Applied Geochemistry, 2001, 16(7): 659-718. |
| [5] | MAO X, XIONG L, HU X, et al. Remediation of ammonia-contaminated groundwater in landfill sites with electrochemical reactive barriers: A bench scale study[J]. Waste Management, 2018, 78: 69-78. doi: 10.1016/j.wasman.2018.05.015 |
| [6] | SIR M, PODHOLA M, PATOCKA T, et al. The effect of humic acids on the reverse osmosis treatment of hazardous landfill leachate[J]. Journal of Hazardous Materials, 2012, 207-208: 86-90. doi: 10.1016/j.jhazmat.2011.08.079 |
| [7] | CHIANESE A, RANAURO R, VERDONE N. Treatment of landfill leachate by reverse osmosis[J]. Water Research, 1999, 33(3): 647-652. doi: 10.1016/S0043-1354(98)00240-1 |
| [8] | LIU D, HUANG K, XIE L, et al. Relation between operating parameters and desalination performance of capacitive deionization with activated carbon electrodes[J]. Environmental Science: Water Research & Technology, 2015, 1(4): 516-522. |
| [9] | YAO Q, TANG H. Occurrence of re-adsorption in desorption cycles of capacitive deionization[J]. Journal of Industrial and Engineering Chemistry, 2016, 34: 180-185. doi: 10.1016/j.jiec.2015.11.004 |
| [10] | BIESHEUVEL P, VAN DER WAL A. Membrane capacitive deionization[J]. Journal of Membrane Science, 2010, 346(2): 256-262. doi: 10.1016/j.memsci.2009.09.043 |
| [11] | BIESHEUVEL P, ZHAO R, PORADA S, et al. Theory of membrane capacitive deionization including the effect of the electrode pore space[J]. Journal of Colloid and Interface Science, 2011, 360(1): 239-248. doi: 10.1016/j.jcis.2011.04.049 |
| [12] | ZHAO R, PORADA S, BIESHEUVEL P, et al. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis[J]. Desalination, 2013, 330: 35-41. doi: 10.1016/j.desal.2013.08.017 |
| [13] | YAO Q, TANG H. Effect of desorption methods on electrode regeneration performance of capacitive deionization[J]. Journal of Environmental Engineering, 2017, 143(9): 04017047. doi: 10.1061/(ASCE)EE.1943-7870.0001245 |
| [14] | LIU D, WANG X, XIE Y, et al. Effect of capacitive deionization on disinfection by-product precursors[J]. Science of the Total Environment, 2016, 568: 19-25. doi: 10.1016/j.scitotenv.2016.05.219 |
| [15] | FANG K, GONG H, HE W, et al. Recovering ammonia from municipal wastewater by flow-electrode capacitive deionization[J]. Chemical Engineering Journal, 2018, 348: 301-309. doi: 10.1016/j.cej.2018.04.128 |
| [16] | 柳青青. 混凝过滤环节及其组合工艺对腐殖酸去除效果研究[D]. 长沙: 湖南大学, 2018. |
| [17] | DEHGHANI M, ZAREI A, MESDAGHINIA A, et al. Production and application of a treated bentonite-chitosan composite for the efficient removal of humic acid from aqueous solution[J]. Chemical Engineering Research and Design, 2018, 140: 102-115. doi: 10.1016/j.cherd.2018.10.011 |
| [18] | CHOI J, LEE H, HONG S. Capacitive deionization (CDI) integrated with monovalent cation selective membrane for producing divalent cation-rich solution[J]. Desalination, 2016, 400: 38-46. doi: 10.1016/j.desal.2016.09.016 |
| [19] | ZORNITTA R, RUOTOLO L A. Simultaneous analysis of electrosorption capacity and kinetics for CDI desalination using different electrode configurations[J]. Chemical Engineering Journal, 2018, 332: 33-41. doi: 10.1016/j.cej.2017.09.067 |
| [20] | CHEN Y, YUE M, HUANG Z, et al. Electrospun carbon nanofiber networks from phenolic resin for capacitive deionization[J]. Chemical Engineering Journal, 2014, 252: 30-37. doi: 10.1016/j.cej.2014.04.099 |
| [21] | GABELICH C J, TRAN T D, SUFFET I H M. Electrosorption of inorganic salts from aqueous solution using carbon aerogels[J]. Environmental Science & Technology, 2002, 36(13): 3010-3019. |
| [22] | LI Y, STEWART T, TANG H. A comparative study on electrosorptive rates of metal ions in capacitive deionization[J]. Journal of Water Process Engineering, 2018, 26: 257-263. doi: 10.1016/j.jwpe.2018.10.021 |
| [23] | LI Y, ZHANG C, JIANG Y, et al. Effects of the hydration ratio on the electrosorption selectivity of ions during capacitive deionization[J]. Desalination, 2016, 399: 171-177. doi: 10.1016/j.desal.2016.09.011 |
| [24] | GIMMI T, ALT-EPPING P. Simulating Donnan equilibria based on the Nernst-Planck equation[J]. Geochimica et Cosmochimica Acta, 2018, 232: 1-13. doi: 10.1016/j.gca.2018.04.003 |
| [25] | TANG W, HE D, ZHANG C, et al. Optimization of sulfate removal from brackish water by membrane capacitive deionization (MCDI)[J]. Water Research, 2017, 121: 302-310. doi: 10.1016/j.watres.2017.05.046 |
| [26] | PORADA S, BRYJAK M, VAN DER WAL A, et al. Effect of electrode thickness variation on operation of capacitive deionization[J]. Electrochimica Acta, 2012, 2(24): 148-156. |
| [27] | PORADA S, ZHAO R, VAN DER WAL A, et al. Review on the science and technology of water desalination by capacitive deionization[J]. Progress in Materials Science, 2013, 58(8): 1388-1442. doi: 10.1016/j.pmatsci.2013.03.005 |
| [28] | HASSANVAND A, CHEN G Q, WEBLEY P A, et al. A comparison of multicomponent electrosorption in capacitive deionization and membrane capacitive deionization[J]. Water Research, 2018, 131: 100-109. doi: 10.1016/j.watres.2017.12.015 |
| [29] | MOUSSAVI G, TALEBI S, FARROKHI M, et al. The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite[J]. Chemical Engineering Journal, 2011, 171(3): 1159-1169. doi: 10.1016/j.cej.2011.05.016 |
| [30] | DU Q, LIU S, CAO Z, et al. Ammonia removal from aqueous solution using natural Chinese clinoptilolite[J]. Separation and Purification Technology, 2005, 44(3): 229-234. doi: 10.1016/j.seppur.2004.04.011 |
| [31] | WANG S, ZHU Z. Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution[J]. Journal of Hazardous Materials, 2006, 136(3): 946-52. doi: 10.1016/j.jhazmat.2006.01.038 |
| [32] | ZHANG X, BAI R. Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules[J]. Journal of Colloid and Interface Science, 2003, 264(1): 30-38. doi: 10.1016/S0021-9797(03)00393-X |
| [33] | ALBERTS J, FILIP Z. Metal binding in estuarine humic and fulvic acids: FTIR analysis of humic acid-metal complexes[J]. Environmental Technology Letters, 2010, 19(9): 923-931. |
| [34] | WANG J, HAN X, MA H, et al. Adsorptive removal of humic acid from aqueous solution on polyaniline/attapulgite composite[J]. Chemical Engineering Journal, 2011, 173(1): 171-177. doi: 10.1016/j.cej.2011.07.065 |
| [35] | MOSSAD M, ZOU L. Study of fouling and scaling in capacitive deionisation by using dissolved organic and inorganic salts[J]. Journal of Hazardous Materials, 2013, 244-245: 387-393. doi: 10.1016/j.jhazmat.2012.11.062 |




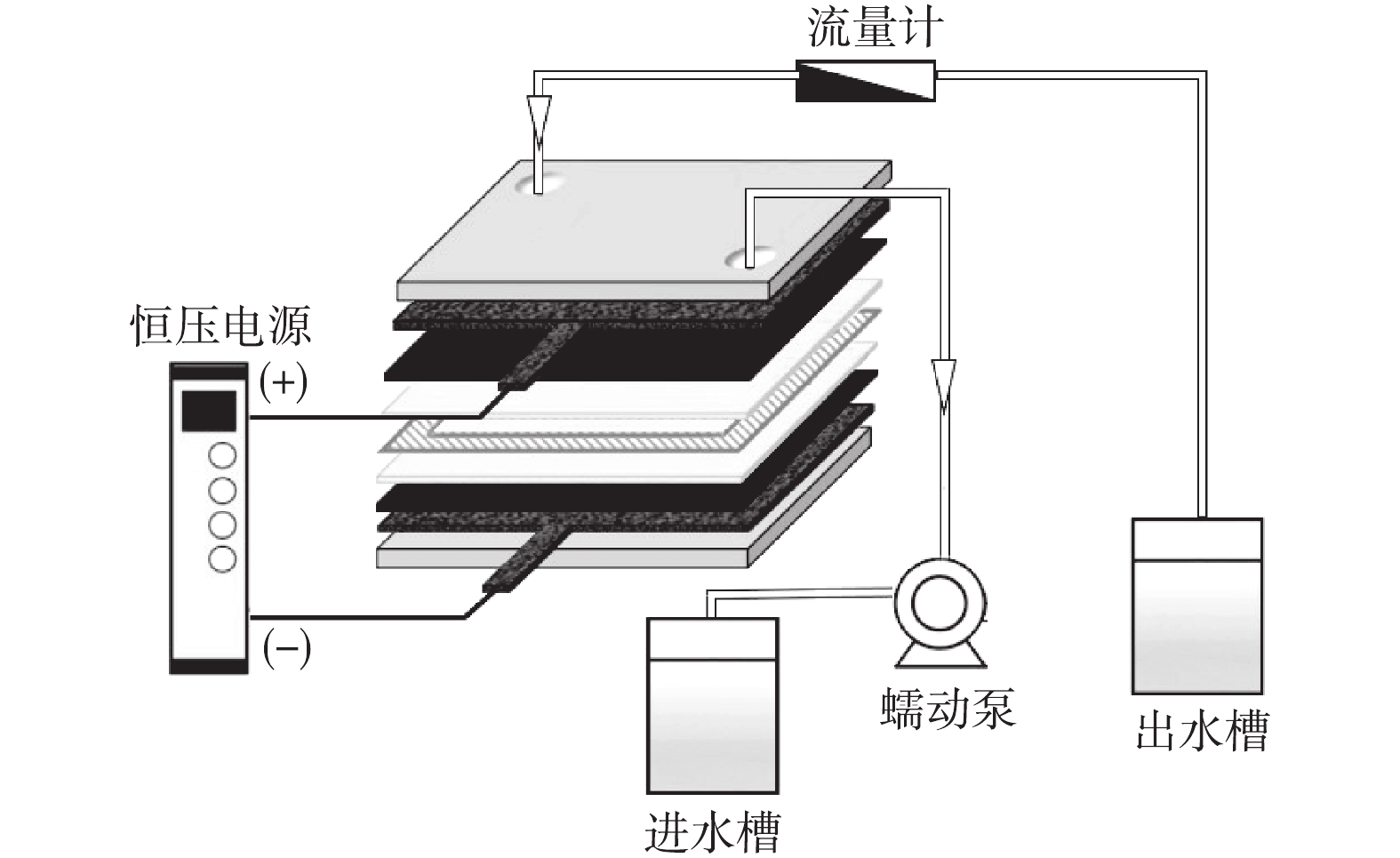
 下载:
下载: 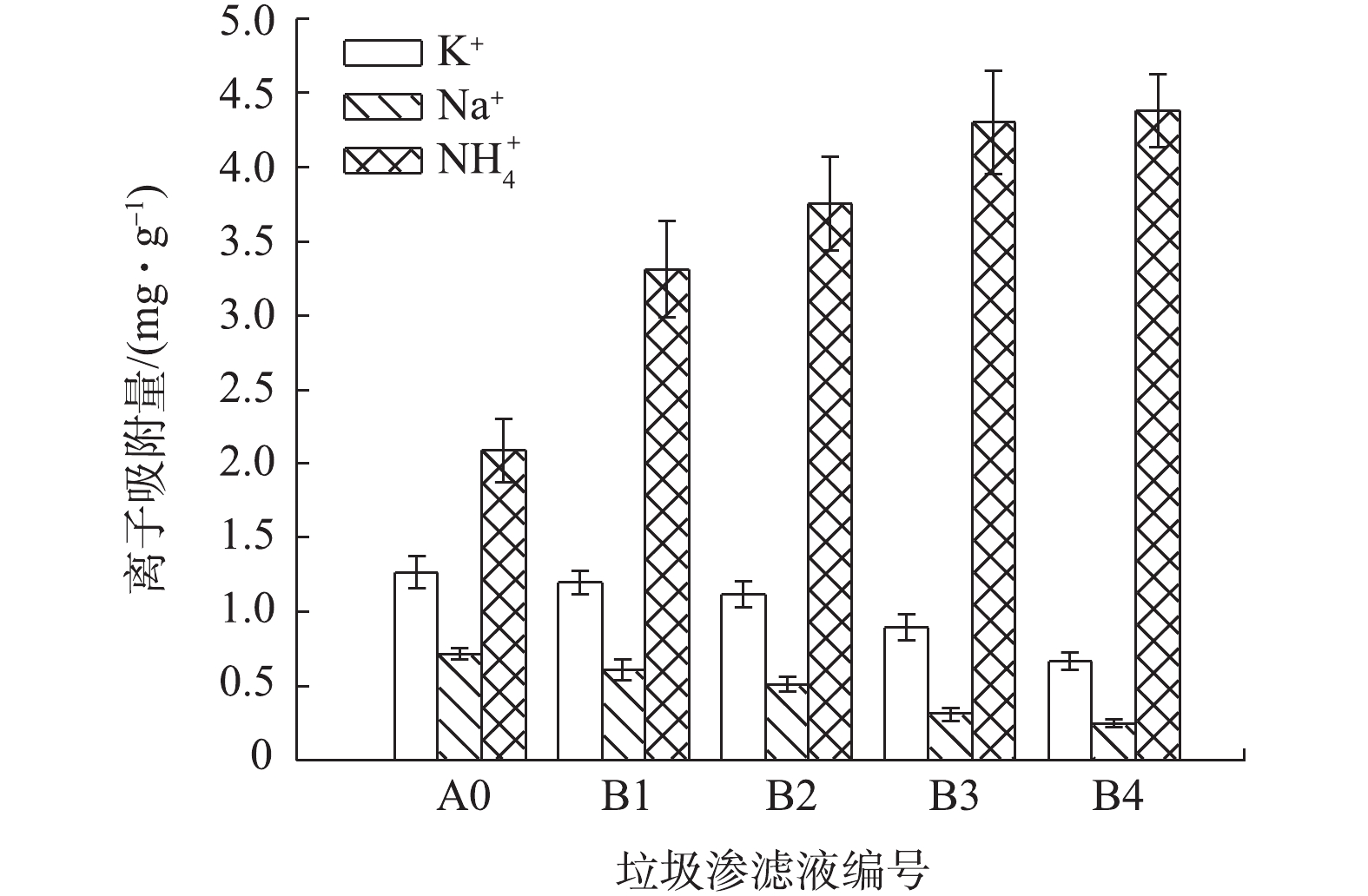












 点击查看大图
点击查看大图