HTML
--> --> -->In contrast to the intensive levels of research having been carried out to address the permafrost C climate feedback (e.g., the Permafrost Carbon Network; http://www.permafrostcarbon.org/), research on permafrost soil N biogeochemistry and the associated release of the potent greenhouse gas (GHG) nitrous oxide (N2O) under a changing climate is strongly lagging behind. Thus far, little is known about the fate of formerly protected organic N during thawing of permafrost soils that store as much as 67 Pg N (Harden et al., 2012). Until recently, the soil N cycle in cold and pristine ecosystems was thought to be largely confined to organic N cycling due to the scarcity of N inputs, slow decomposition at low temperatures and high competition for bioavailable N between biota (van Cleve and Alexander, 1981; Schimel and Bennett, 2004). Thus, it has been postulated for a long time that N2O emissions from permafrost soils are low as a result of limited amounts of inorganic N (Rodionow et al., 2006; Chapin et al., 2011). However, over the last decade, a growing number of studies have reported very high N2O emissions from permafrost soils, which are in a comparable range as observed for tropical forests or agricultural ecosystems (e.g., Repo et al., 2009; Elberling et al., 2010; Marushchak et al., 2011; Palmer et al., 2012; Abbott and Jones, 2015; Voigt et al., 2017a; Liu et al., 2018; Wilkerson et al., 2019). In addition, the few available studies on experimental warming of permafrost soils hint at a stimulation of N2O emissions by temperature (Chen et al., 2017; Voigt et al., 2017a, b; Cui et al., 2018). The potential significance of inorganic N cycling and N2O release in permafrost soils can be illustrated by a simple calculation. If 10% of the organic N stored in permafrost soils (i.e., 6.7 Pg N) is released between the present day and the year 2100, as it has been estimated for C release (Schuur et al., 2015), and only 1% is emitted as N2O (67 Tg N2O-N), just like the IPCC’s default N2O emission factor for N mineralized from mineral soils (IPCC, 2006), this would be equivalent to 10 times the global annual rate of N2O emissions from soils under natural vegetation [6.6 Tg N2O-N yr?1 (Ciais et al., 2013)]. Consequently, there is an urgent need to better understand N biogeochemistry and associated gaseous N emissions in permafrost soils under the auspices of a warming climate.
Therefore, NIFROCLIM combines (1) research on the quality and quantity of N in soil organic matter; (2) molecular measurements of the abundance and activity of soil microbes involved in the N cycle; (3) isotope-based quantitative biogeochemical process studies; and (4) measurements of N gas (NO, N2O, N2) production in soil and the exchange at the soil–atmosphere interface in an interdisciplinary approach.
NIFROCLIM was launched with a kickoff meeting and a first campaign in the target region of Mohe County, Northeast China, in July 2019. The sampling concept in the research area encompasses (1) high-resolution soil and gas sampling in vertical soil profiles from the soil surface to the active layer and into the upper layers of the permafrost; (2) soil and vegetation sampling as well as GHG flux measurements across topographic landscape transects covering different upland forests and lowland bogs; and (3) investigations of climate change effects by using open top chambers (OTCs) to simulate warming.
 Figure1. (a) Map of permafrost-affected areas in China (Cheng and Jin, 2013) showing the Eurasian permafrost in the northeast. (b) Geographical location of the experimental region.
Figure1. (a) Map of permafrost-affected areas in China (Cheng and Jin, 2013) showing the Eurasian permafrost in the northeast. (b) Geographical location of the experimental region.Scientific infrastructure has been established at four sites in the upper, middle and lower reaches of Fukuqi River to investigate permafrost N biogeochemistry and soil–atmosphere gas exchange (Fig. 1b). At the most extensively equipped Site 2, the effects of long-term warming are studied in situ, using OTC warming platforms in different habitats (B. fruticosa and L. palustre communities of ombrotrophic bogs) against controls outside the OTCs. The OTCs increase the soil temperature (5 cm depth) and active layer depth on average by 2°C and 7 cm, respectively, during the growing seasons (Cui et al., 2018). At the same site, fully automated static chamber measuring systems with 12 chambers and an eddy covariance system allow for online quantification of CO2, CH4 and N2O fluxes at hourly temporal resolution [Figs. 2a and b (Liu and Zheng, 2019)] from March to November. To further address landscape-scale flux variations, manual static and dynamic chamber measuring systems are used to detect the soil–atmosphere CO2, CH4, N2O and NOx fluxes at daily to sub-weekly temporal resolution at all sites in the catchment (overall > 100 flux chambers) (Figs. 2c and d) (Valente et al., 1995; Zhang et al., 2014).
 Figure2. The measuring systems used to quantify biosphere–atmosphere gas fluxes. (a) Automated static translucent chambers for measuring CO2, CH4 and N2O fluxes. (b) Eddy covariance system for measuring CH4, CO2 and water vapor exchanges. (c) Manual static opaque chambers for measuring soil respiration, CH4 and N2O fluxes. (d) Manual dynamic opaque chambers for measuring NOx fluxes.
Figure2. The measuring systems used to quantify biosphere–atmosphere gas fluxes. (a) Automated static translucent chambers for measuring CO2, CH4 and N2O fluxes. (b) Eddy covariance system for measuring CH4, CO2 and water vapor exchanges. (c) Manual static opaque chambers for measuring soil respiration, CH4 and N2O fluxes. (d) Manual dynamic opaque chambers for measuring NOx fluxes.Air samples from manual chamber measurements are analyzed by gas chromatography and a chemoluminescence NO-NO2-NOx analyzer in the field laboratories at Tuqiang—a town at the outlet of the catchment (Fig. 1b). The laboratories at Tuqiang also offer basic equipment for extraction and stabilization of DNA from soils, which is needed to analyze the soil microbiome and to track soil microbial N transformations via molecular microbiological approaches. Moreover, at Tuqiang, soil samples including intact frozen soil cores are processed and labeled for stable isotope studies (Fig. 3) in order to quantify soil gross N turnover rates [protein depolymerization, ammonification, nitrification, microbial immobilization, biological N fixation (BNF)].
 Figure3. Permafrost soil sampling. (a) Frozen ground at ~20 cm depth in July 2019 at a wetland site with soil temperature measurement showing 4.7°C at 6.5 cm depth and 2.0°C at 14 cm depth. (b) Intact frozen soil core. (c) Labeling of frozen soil for stable isotope studies.
Figure3. Permafrost soil sampling. (a) Frozen ground at ~20 cm depth in July 2019 at a wetland site with soil temperature measurement showing 4.7°C at 6.5 cm depth and 2.0°C at 14 cm depth. (b) Intact frozen soil core. (c) Labeling of frozen soil for stable isotope studies.Topography, climate, land use, soil (e.g., soil texture, bulk density, pH, total N and organic C contents), vegetation and management information are collected at the site or catchment scale to run the process-oriented hydrobiogeochemical model CNMM-DNDC [Fig. 4 (Zhang et al., 2018)]. Soil variables (e.g., temperature, moisture, dissolved organic C and inorganic N concentrations), soil–atmosphere gas fluxes, stream discharge and related dissolved C and N losses during runoff processes are measured to validate the model at the grid (150 m × 150 m) or catchment scale. The hydrobiogeochemical model CNMM-DNDC will finally be used to upscale gaseous and dissolved C and N losses at the catchment scale, as well as to conduct scenario analyses of climate change to evaluate the influences of permafrost thawing on vegetation succession, permafrost N cycling and N2O emissions under the Representative Concentration Pathways.
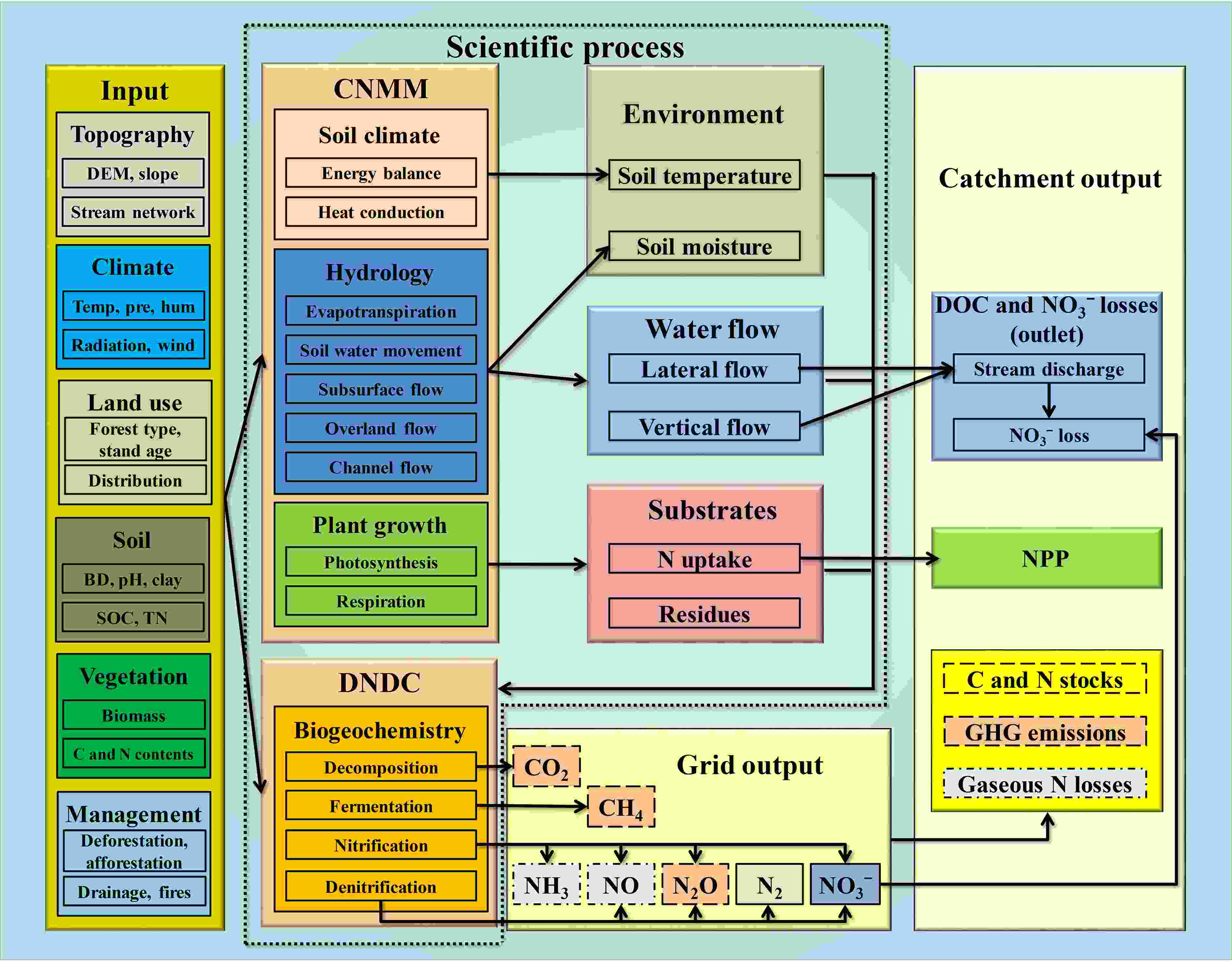 Figure4. The CNMM-DNDC model structure [Reprinted from Zhang et al. (2018)].
Figure4. The CNMM-DNDC model structure [Reprinted from Zhang et al. (2018)].By combining the expertise of the researchers involved, the project is targeted at providing a comprehensive picture of the N cycle and N balances for the main ecosystems present in the permafrost region of Northeast China. Through the interdisciplinary cooperation between atmospheric physicists, soil biogeochemists, microbiologists, plant physiologists and soil scientists, NIFROCLIM aims at a holistic understanding of the permafrost soil N cycle and associated N2O emissions at a catchment scale under the auspices of climate change, and thus at providing a significant contribution towards a better prediction of potential N to climate feedbacks of permafrost ecosystems. In this context, NIFROCLIM is also seen as a platform for international cooperation on permafrost research in China and beyond.
Acknowledgements. Funding for the project is gratefully acknowledged and has been provided by the NSFC (Grant No. 41861134029) and DFG (Grant Nos. DA1217/4-1 and SCHL446/41-1).
