摘要/Abstract
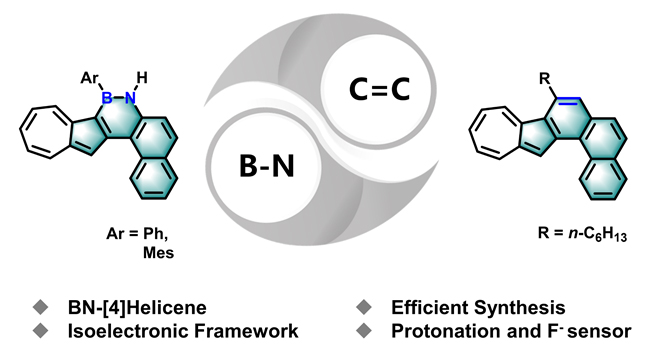
本工作设计合成了分别含有B—N键和C=C键的薁基[4]螺烯类分子1a/1b和2, 其中B—N键和C=C键互为等电子体. 紫外-可见吸收光谱、电化学循环伏安测试和理论计算结果表明B—N键可以调控共轭骨架的电子结构及芳香性. 单晶结构表明1a具有螺旋几何构型, 晶体中存在P和M两种对映异构体. B—N键具有部分双键性质, 硼氮六元环具有一定的芳香性. 大位阻基团2,4,6-三甲基苯基(Mes)使得1b在三氟乙酸(TFA)作用下不会发生类似于1a的脱硼化, 而是发生和2相似的可逆质子响应, B—N键对薁单元的质子响应性质无明显影响. 三配位的硼原子可以进一步和氟离子配位, 使得1a对氟离子有明显的选择性响应, 而1b则因大位阻的Mes取代基的存在对氟离子无明显的响应性. 本工作报道了新型薁基硼氮杂螺烯及全碳螺烯分子, 为薁基多环芳烃的“自下而上”合成及性质研究提供了参考.
关键词: 薁, 硼氮杂多环芳烃, 螺烯, 质子化
Azulene is a nonalternant and nonbenzenoid hydrocarbon with bright blue color and a dipole moment of 1.08 D, and has received increasing attention due to its unique electronic structure and physicochemical properties. Herein, we report the design and synthesis of two types of azulene-based [4]helicene 1a/1b and 2 that contain isoelectronic B—N and C=C units at the electron-rich 1-position of azulene unit, respectively. Formation of the helical scaffolds is executed by the introduction of boron and alkyne to flexible biaryl precursors, where the Lewis acidic boron and alkyne were employed as “glue” to join two subunits into fully fused scaffolds via electrophilic boronation and platinum-catalyzed cycloisomerization of alkyne at the 1-position of azulene unit, respectively. All of azulene-based helicenes were investigated by ultraviolet visible (UV-vis) absorption spectra, cyclic voltammetry (CV) measurements and density functional theory (DFT) calculations. Additionally, 1a was further characterized by single crystal structure analysis. The results suggest that the introduction of B—N unit changed the electronic structure of the conjugated aromatic framework, leading to a narrow HOMO-LUMO gap. Moreover, the B—N unit also affects the aromaticity of the π-system as revealed by nucleus-independent chemical shift (NICS) via time-dependent density functional theory (TD-DFT) calculation. The single crystal structure analysis demonstrates that 1a has a helically twisted framework and Plus (P)/Minus (M) enantiomers. However, the Gibbs activation energy (ΔG≠(T)) of the enantiomerization at room temperature is too low to separate two enantiomers by chiral high performance liquid chromatography (HPLC). Furthermore, the B—N unit exhibits partial double bond character and the BN-containing six-membered ring shows weak aromaticity. 1a with a phenyl group exhibits the deboronization upon addition of trifluoroacetic acid (TFA) as well as a specific sensing behavior to fluoride ion. However, 1b shows no deboronization upon addition of TFA and no sensing behavior to fluoride ion due to its steric hindered mesityl (Mes) group, but has a reversible stimuli-responsiveness with acid and base, this proton-responsiveness is similar to all-carbon analogue 2.
Key words: azulene, BN-heteroaromatic, helicene, protonation
PDF全文下载地址:
点我下载PDF
