摘要/Abstract
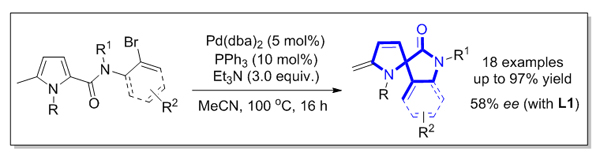
基于迁移插入策略, 研究了钯催化吡咯环内共轭双键的分子内Heck反应. 在温和的反应条件下, 以良好至优异的收率合成了一系列含有二氢吡咯及2-吲哚酮结构的螺杂环化合物. 同时, 以八氢联萘酚衍生的亚磷酰胺为手性配体, 初步探索该对映选择性反应, 获得中等水平的对映体过量值.
关键词: 钯, Heck反应, 吡咯, 吲哚酮, 对映选择性
3,2’-Spiropyrrolidine-2-oxindole scaffold is found as a key unit in a number of pharmaceutical candidates and nature products. It is highly desirable to develop efficient synthetic strategies to access such a structural scaffold. Recently, dearomatization reactions have received considerable attention as an efficient and straightforward synthetic method to convert readily available aromatic compounds to three-dimensional organic molecules. Amongst them, the transition-metal-catalyzed dearomative functionalization of endocyclic C=C bonds of aromatic compounds initiated by dearomative migratory insertion has been extensively established. A number of dearomative Heck reactions, reductive Heck reactions, and domino Heck-anionic capture sequences have been developed. Nevertheless, the present studies are mainly focused on the dearomatization reactions of indoles and furans. In contrast, there are rare examples reported for the dearomatization of pyrroles. In this communication, we report a palladium-catalyzed intramolecular Heck reaction of the endocyclic conjugated C=C bonds of C2-tethered pyrroles through an initial migratory insertion to formal conjugate diene and subsequent hydride elimination. A range of 3,2’-spiropyrrolidine-2-oxindole derivatives are obtained in good to excellent yields, showing broad substrate scope. In addition, a preliminary study of enantioselective reaction implies that the target product could be obtained in moderateee under the help of a H8-BINOL-based chiral phosphoramidite ligand. A general procedure for this dearomative Heck reaction is depicted as the follows: to a dried Schlenk tube were added pyrrole substrate 1 (0.20 mmol), Pd(dba)2 catalyst (5.8 mg, 0.010 mmol), and PPh3 ligand (5.2 mg, 0.020 mmol) under N2 atmosphere. 2.0 mL of MeCN solvent and 83 µL of Et 3N were then introduced through a syringe and the Schlenk tube was sealed using a Teflon cap. The resulting reaction mixture was stirred at 100 ℃ for 16 h. After the reaction was completed (monitored by TCL), the mixture was concentrated under vacuum and the residue was purified by flash column chromatography on silica gel to afford the product 2.
Key words: palladium, Heck reaction, pyrrole, oxindole, enantioselectivity
PDF全文下载地址:
点我下载PDF
