摘要/Abstract
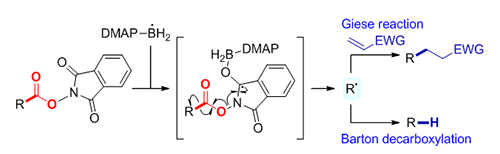
邻苯二甲酰亚胺羧酸酯的自由基脱羧是产生碳自由基的一类重要方法. 开发了一类路易斯碱-硼自由基促进的邻苯二甲酰亚胺羧酸酯的自由基脱羧新方式, 产生的烷基自由基经过加成到缺电子烯烃上或者进一步还原得到Giese反应和Barton脱羧产物. 该反应条件温和, 适用范围广泛, 多种一级、二级和三级烷基羧酸包括具有生物活性的天然产物和药物分子都能够较好地适用, 为邻苯二甲酰亚胺羧酸酯的自由基脱羧提供了无过渡金属参与的具有普适性的新方式.
关键词: 对二甲氨基吡啶-硼自由基, 自由基脱羧反应, Giese反应, Barton脱羧还原, 自由基化学
Decarboxylation of N-hydroxyphthalimide (NHPI) esters represents a powerful tool to generate carbon radicals, which has wide applications in the construction of C—C bonds and C—X bonds. Traditionally, the radical decarboxylation of NHPI esters has been enabled by transition-metal catalysis and photoredox catalysis. Recently, several visible light-mediated photosensor-free decarboxylation reactions have been reported with the use of special electron-donor systems. While notable, it’s still highly desirable to develop transition-metal-free, mild, and general methods to realize the radical decarboxylation of NHPI esters. Herein, we report 4-dimethylaminopyridine (DMAP)-boryl radical enabled Giese reaction and Barton decarboxylation of NHPI esters. The reaction starts from the generation of DMAP-boryl radical in the presence of a radical initiator, which then adds specifically to the carbonyl oxygen atom of NHPI ester 2, followed by β-fragmentation to give a nucleophilic carbon radical intermediate. Addition of the carbon radical to electron-deficient alkenes affords the Giese reaction product 4. On the other hand, hydrogen atom transfer from thiol to the nucleophilic carbon radical results in the Barton decarboxylation products 5. The reactions exhibit a broad substrate scope and excellent functional group tolerance. NHPI esters of primary, secondary, and tertiary alkyl carboxylic acids, including bio-active natural products and drugs, proceed smoothly to give the corresponding products in moderate to good yields. A variety of electron-deficient alkenes, such as vinyl esters, vinyl amides and vinyl sulphones, can be used as the Michael acceptors. A general procedure for the Giese reaction is as following: a solution of NHPI ester 2 (0.5 mmol), 4-dimethylaminopyridine-borane (0.6 mmol), AIBN (0.1 mmol) and electron- deficient alkenes 3 (0.4 mmol) in toluene (4.0 mL) was stirred at 80 ℃ for 4 h under nitrogen atmosphere. After evaporation of solvent, the crude residue was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford Giese reaction product 4. A general procedure for the Barton decarboxylation is as following: a solution of NHPI ester 2 (0.5 mmol), 4-dimethylaminopyridine-borane (0.55 mmol), TBHN (0.1 mmol) and PhSH (0.1 mmol) in benzotrifluoride (5.0 mL) was stirred at 80 ℃ for 1 h under nitrogen atmosphere. After evaporation of solvent, the crude residue was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford decarboxylative reduction product 5.
Key words: 4-dimethylaminopyridine-boryl radical, radical decarboxylation, Giese reaction, Barton decarboxylative reduction, radical chemistry
PDF全文下载地址:
点我下载PDF
