摘要/Abstract
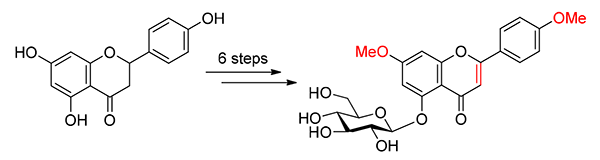
7,4'-二甲氧基洋芹素-5-O-葡萄糖苷作为珍贵中药白木香的有效成分具有抑制LPS诱导巨噬细胞生成NO活性.由于5-位羟基具有较强的分子内氢键,5-位氧苷的黄酮类化合物在传统的糖苷化条件不能高效合成.以价廉易得的柚皮素和D-葡萄糖为原料,经选择性羟基保护、硼氢化钠还原、相转移催化下的糖苷化、2,3-二氯-5,6-二氰基对苯醌(DDQ)氧化等6步反应,以36.0%的总收率完成了7,4'-二甲氧基洋芹素-5-O-葡萄糖苷的化学合成,为该化合物进一步的生物活性研究奠定了物质基础.
关键词: 白木香, 7,4'-二甲氧基洋芹素-5-O-葡萄糖苷, 黄酮, 化学合成
As the active component of precious Chinese medicine Aquilaria sinensis, 7,4'-dimethylapigenin-5-O-glycoside showed inhibitory activity for nitric oxide (NO) production by activated RAW 264.7 cells. Because of the strong intramolecular H-bond, the 5-O-glucosidic linkage in flavonoids could not be efficiently constructed via conventional glycosylation method. In this paper, the efficient chemical synthesis of 7,4'-dimethylapigenin-5-O-glycoside has been achieved for the first time starting from commercially available naringenin and D-glucose via a linear reaction sequence of 6 steps with the overall yield of 36.0%, wherein selective hydroxy protecting, reduction with sodium borohydride, glycosylation under phase transfer catalytic condition, oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and other reactions were used. This work definitely laid the foundation for the further pharmacological study of this natural compound.
Key words: Aquilaria sinensis, 7,4'-dimethylapigenin-5-O-glycoside, flavonoids, chemical synthesis
PDF全文下载地址:
点我下载PDF
