摘要/Abstract
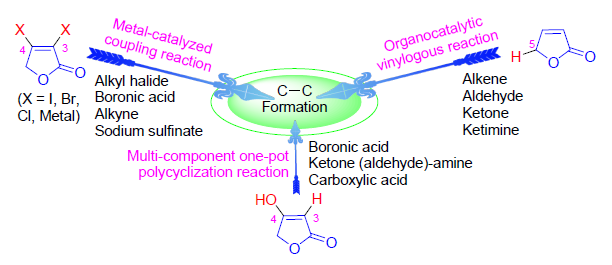
2(5H)-呋喃酮具有多个反应位点,同时其骨架广泛存在于许多天然产物的结构中,因此2(5H)-呋喃酮的衍生化反应具有重要的研究意义.一些简单的2(5H)-呋喃酮分子,如3-位(或4-位)卤代的2(5H)-呋喃酮、5-位无取代基的2(5H)-呋喃酮以及4-羟基-2(5H)-呋喃酮及其衍生物等,可以与有机金属化合物、卤代烃、有机硼化合物、不饱和烃以及不饱和C=X(X=O、N)等多种试剂作用,分别在2(5H)-呋喃酮骨架的3-位、4-位、5-位等不同位置上构建C-C键.鉴于此,以反应试剂为分类依据,综述了近年来基于2(5H)-呋喃酮骨架的C-C成键反应,总结了它们在有机合成方法学中及其生物活性化合物合成应用中的新进展,并指出进一步实现2(5H)-呋喃酮C-C成键反应的绿色化及其高效多环化利用是未来的重要研究方向.
关键词: 2(5H)-呋喃酮, C-C键构建, 金属催化偶联反应, 有机小分子催化Vinylogous型反应, 多环化反应
2(5H)-Furanone contains several reaction points, and its structure unit exists in a number of nature products, which makes the researches on the derivatizations of 2(5H)-furanone important. Some 2(5H)-furanone compounds, such as 3-(or 4-) halo-2(5H)-furanone, 5-nonsubstitued 2(5H)-furanone, 4-hydroxy-2(5H)-furanone and their derivatives, can react with organometallic reagents, alkyl halides, organoboron compounds, unsaturated hydrocarbons and unsaturated C=X (X=O, N) compounds, forming C-C bond at 3-, or 4-, or 5-position of 2(5H)-furanone, respectively. According to the different types of reagents, the C-C bond formation reactions based on 2(5H)-furanone synthons are reviewed, and their recent progress in organic synthesis methodology and application of bioactive compounds is summarized. In the future, it is important to make the 2(5H)-furanone C-C bond formation reaction more greener and efficiently used in polycyclization reaction.
Key words: 2(5H)-furanone, C-C bond formation, metal-catalyzed coupling reaction, organocatalytic vinylogous reaction, polycyclization reaction
PDF全文下载地址:
点我下载PDF
