

江南大学 生物工程学院 工业生物技术教育部重点实验室,江苏 无锡 214122
收稿日期:2020-10-04;接收日期:2021-01-12
基金项目:国家自然科学基金(Nos.32070035,31770058),国家重点研发计划项目(No.2018YFA0900300),江苏省自然科学基金(No.BK20181205),宁夏回族自治区重点研发计划(No.2019BCH01002),国家轻工技术与工程一流学科自主课题(No.LITE2018-06)资助
作者简介:饶志明??博士、江南大学教授、博士生导师、粮食发酵工艺与技术国家工程实验室副主任。入选中组部“****”科技创新领军人才、教育部新世纪优秀人才、江苏省****基金获得者、江苏省青蓝工程中青年学术带头人等。受聘为国家863首席专家、中国发酵工业委员会委员、江南大学轻工技术与工程一流学科方向课题组长。近五年以责任作者在Science Advances、Nature Communications、Green Chemistry、Metabolic Engineering、《中国科学》和《生物工程学报》等期刊发表高水平研究论文150余篇,带领团队成功研发出多个位居世界领先水平的重大高值化氨基酸等产品的生产菌株并实现产业化,取得显著社会经济和生态效益.
摘要:谷氨酸棒杆菌Corynebacterium glutamicum作为一般被认为具有生物安全性的一种模式工业微生物,不仅在发酵工业中成功用于大规模生产氨基酸,而且具有合成多种新型化学品的潜力。谷氨酸棒杆菌菌株在生产化合物时,经常会受到各种逆境条件的胁迫,从而降低细胞活力和生产性能。合成生物学的发展为提高谷氨酸棒杆菌的鲁棒性提供了新的技术手段。本文总结了谷氨酸棒杆菌应对发酵过程中各种胁迫的耐受机制。同时,重点介绍提高谷氨酸棒杆菌底盘细胞鲁棒性和耐受性的合成生物学新策略,包括挖掘新的抗逆元件、改造转录调控因子、利用适应性进化策略挖掘抗逆功能模块等。最后,从生物传感器、转录调控因子的筛选和设计、多种调控元件利用等方面对提高谷氨酸棒杆菌底盘细胞鲁棒性进行了展望。
关键词:谷氨酸棒杆菌底盘细胞鲁棒性耐受机制合成生物学
Advances in stress tolerance mechanisms and synthetic biology for the industrial robustness of Corynebacterium glutamicum
Meijuan Xu, Chunyu Shangguan, Xin Chen, Xian Zhang, Taowei Yang, Zhiming Rao


Key Laboratory of Industrial Biotechnology, Ministry, of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
Received: October 4, 2020; Accepted: January 12, 2021
Supported by: National Natural Science Foundation of China (Nos. 32070035, 31770058), National Key Research and Development Program of China (No. 2018YFA0900300), Natural Science Foundation of Jiangsu Province, China (No. BK20181205), Ningxia Hui Autonomous Region Key Research and Development Program, China (No. 2019BCH01002), National Light Industry Technology and Engineering First-Class Subject Independent Project (No. LITE2018-06)
Corresponding author: Zhiming Rao. Tel/Fax: +86-510-85916881; E-mail: raozhm@jiangnan.edu.cn.
Abstract: As a model industrial host and microorganism with the generally regarded as safe (GRAS) status, Corynebacterium glutamicum not only produces amino acids on a large scale in the fermentation industry, but also has the potential to produce various new products.C.glutamicum usually encounters various stresses in the process of producing compounds, which severely affect cell viability and production performance. The development of synthetic biology provides new technical means for improving the robustness of C.glutamicum. In this review, we discuss the tolerance mechanisms of C. glutamicum to various stresses in the fermentation process. At the same time, we highlight new synthetic biology strategies for boosting C.glutamicum robustness, including discovering new stress-resistant elements, modifying transcription factors, and using adaptive evolution strategies to mine stress-resistant functional modules. Finally, prospects of improving the robustness of engineered C. glutamicum strains ware provided, with an emphasis on biosensor, screening and design of transcription factors, and utilizing the multiple regulatory elements.
Keywords: Corynebacterium glutamicumchassis cellrobustnesstolerance mechanismsynthetic biology
利用工业微生物细胞工厂合成天然产物[1]、生物燃料[2]和化学品[3]是一种可持续可发展的方法。为了建立具有高产量、高效率和高转化率的高效微生物细胞工厂,研究人员已经开发了许多代谢工程策略,包括筛选或改造关键酶克服限速步骤[4]、增加前体和辅因子供应[5]、微调基因表达和阻断竞争途径等[6]。尽管这些策略取得了长足的进步,但微生物菌株在工业应用时经常面临各种胁迫条件,严重制约了细胞活力和生产性能。例如,微生物细胞工厂在大规模发酵过程中不断受到由代谢失衡、基因和表型不稳定性以及恶劣的外部环境引起的干扰[7];同时,细胞内环境和中间体浓度的波动会影响微生物代谢平衡[8]。另外,在实验室规模和商业规模下,苛刻的细胞外环境,例如高温、低pH和代谢产物毒性等,要求宿主具有鲁棒性才能更好地生产产物[9]。因此,提高宿主鲁棒性成为微生物底盘细胞工厂设计和建造中的主要考虑因素之一。
谷氨酸棒杆菌Corynebacterium glutamicum不仅是绿色生物制造氨基酸产业的核心菌株,更是研究透彻的模式生物之一。50多年来,通过经典育种、代谢工程、系统以及合成生物学方法对这种放线菌纲的产氨基酸菌株进行改造,谷氨酸棒杆菌已被设计用于生产氨基酸、有机酸、聚合物前体、芳香族化学品和蛋白质等[10-12]。合成生物学被定义为工程原理在生物学上的应用,旨在使设计基因编码生物系统的过程更加稳定、高效和系统化[13]。因此,为改善微生物细胞抵抗胁迫环境的能力,合成生物学作为有效工具可以为细胞提供元件或部件来帮助微生物抵抗压力。在这篇综述中,我们总结了谷氨酸棒杆菌抗各种环境胁迫机制以及利用合成生物学改善遗传和表型稳定性来增强其对环境压力耐受性的案例。此外,我们讨论了谷氨酸棒杆菌提高工业鲁棒性的各种合成生物学策略。
1 谷氨酸棒杆菌耐受胁迫机制1.1 谷氨酸棒杆菌对热胁迫的耐受机制在发酵过程中微生物会因菌体生长和代谢而释放大量热能。当发酵温度超过一定范围时,菌种新陈代谢加快而容易发生早衰,严重影响目标代谢物的产率。热胁迫对微生物生长的影响可以归结为以下几个方面:抑制细胞分裂和生长、降低细胞活力、改变细胞形态、破坏细胞膜和线粒体结构及细胞骨架的完整性、抑制蛋白质的合成、损坏染色体结构[14]。
谷氨酸棒杆菌对非最佳生长温度的适应机制包括在40 ℃以上诱导的热休克反应和在20 ℃以下诱导的冷休克反应,两者都涉及不同的伴侣分子。C.glutamicum有7种伴侣蛋白表现出对热休克的显著诱导作用,即DnaK、GroEL1、GroEL2、ClpB、ClpC、ClpP和GrpE。其中DnaK和GroEL尝试重新折叠错误折叠的蛋白质,而ClpC和ClpP介导剧烈次级反应[15]。除分子伴侣外,C.glutamicum ATCC 13032基因组中肽基-脯氨酰顺/反异构酶FkpA在体外能延迟柠檬酸合酶的热聚集,扩大了柠檬酸合酶活性的温度范围,低温下促进柠檬酸合酶活性。FkpA减慢蛋白质聚集的能力可能有助于减少诱导的伴侣蛋白表达的时间间隔[16]。当环境温度提高时,特殊转录因子被激活,产生大量热激蛋白,维持细胞内的正常代谢。在C.glutamicum中,通过改变Sigma因子sigH基因表达水平和蛋白活性来介导特定热休克基因的调控。sigH基因表达被热激激活后导致clpC和clpP1P2基因的转录激活[17]。Matsutani等[18]发现新分离的C.glutamicum N24与其他相关物种菌株相比,具有谷胱甘肽依赖性醛脱氢酶(N24_2410)、分选酶同源物(N24_2648和N24_3002) 以及与脂质合成相关的基因操纵子(N24_1290-N24_1292);他们推测这些基因通过帮助构建细胞表面结构和合成脂质从而提高了菌株耐热性。
1.2 谷氨酸棒杆菌对高渗透胁迫的耐受机制发酵过程中,发酵液中高浓度的营养物质和产物都可能引起渗透压的变化,从而影响细胞的生产性能。如图 1所示,为了规避环境中高渗条件的有害影响,C.glutamicum通过BetP、EctP、ProP、LcoP相容性溶质摄取系统积累相容性溶质如甜菜碱、脯氨酸、四氢嘧啶和哌啶酸,以确保水持续流入细胞质或自身从头合成相容性溶质如脯氨酸、海藻糖和谷氨酰胺[19]。此外,C.glutamicum还进化出MtrAB双组分信号转导系统防御机制。MtrAB双组分系统可以调节参与细胞壁生物合成的两个基因mepA和nlpC的表达[20]。当细胞通过通道蛋白CglK吸收K+时,K+积累会使MtrB自磷酸化从而使C.glutamicum激活相容性溶质摄取系统中的3种转运蛋白Bet、ProP和LcoP以及机械敏感通道蛋白MscL[21]。
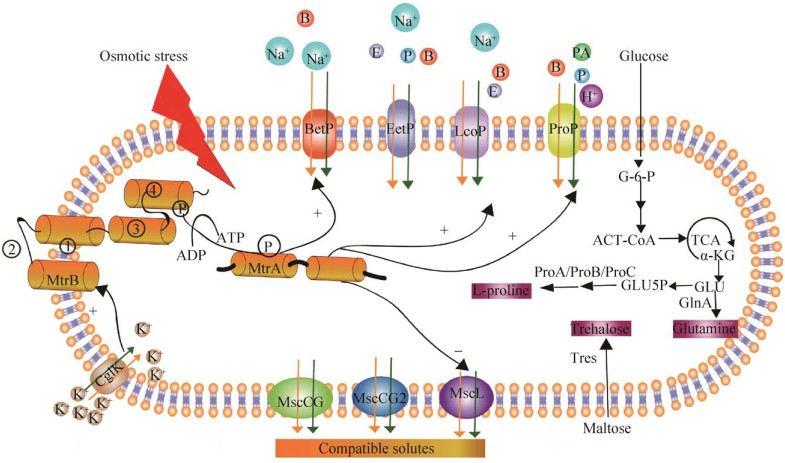 |
| 图 1 谷氨酸棒杆菌对高渗透压胁迫的耐受机制 Fig. 1 Mechanisms of C.glutamicum responds to osmotic stress. G-6-P: glucose-6-phosphate; ACT-CoA: acetyl-CoA; α-KG: α-ketoglutarate; GLU5P: γ-glutamyl phosphate; ProA: glutamate-5-semialdehyde dehydrogenase; ProB: γ-glutamyl kinase; ProC: pyrroline-5-carboxylate reductase; GlnA: glutamine synthetase; Tres: trehalose synthase; orange ball: betaine; green ball: pipecolic acid; blue ball : proline; light purple ball: ectoine; membrane channel proteins: MscCG, MscCG2, MscL; uptake systems for compatible solutes: Betp, Ectp, Lcop, Prop; MtrA: the soluble response regulator; MtrB: the membrane-bound histidine kinase. |
| 图选项 |
对于低渗胁迫,细菌细胞抵抗低渗胁迫的第一道防线是由作为应急阀门的机械敏感通道提供的。在谷氨酸棒杆菌基因组中,存在两个MscS类型机械敏感通道MscCG、MscCG2和一个MscL样通道CgMscL[22]。谷氨酸棒状杆菌通道通过响应膜张力变化而打开,以释放出相容性溶质,从而平衡过多的水分流入以防止细胞裂解[23]。
1.3 谷氨酸棒杆菌对pH波动胁迫的耐受机制在有氧和厌氧发酵生产过程中,谷氨酸棒杆菌会面对如发酵环境和自身产生的以及外部添加的各种类型的酸类物质。胞内酸环境会生成活性氧(Reactive oxygen species,ROS)、破坏芬太尼反应和铁饥饿反应等,从而扰乱多种代谢途径[24-27]。
面对pH波动环境,谷氨酸棒杆菌进化了多种策略来维持细胞内pH的稳态,如图 2所示。Xu等[25]发现酸性条件下C.glutamicum的过氧化氢酶(KatA)、过氧化物酶(Mpx) 和保护DNA蛋白(Dps) 协同介导了细胞内ROS清除。同时还发现两个铜伴侣基因cg1328和cg3292参与酸胁迫条件下的细胞存活[25]。此外,硫代谢转录调控因子McbR可通过转录阻遏物抑制硫同化、减少含硫中间体的积累来提高耐酸性[25]。转运通道蛋白CglK可介导K+积累保持细胞质的中性pH,改善C.glutamicum的生长[28]。Jakob等[29]分析了经过长期乳酸适应的谷氨酸棒杆菌的基因表达谱,发现缺乏sigma因子SigB可导致细胞对低pH的敏感性明显增加。酶复合物F1-F0-ATPase利用ATP水解的能量可将质子排出,在耐酸性中起重要作用[29]。而C.glutamicum中Na+/H+反转运蛋白Mrp1、Mrp2、Nhap,在赋予C.glutamicum的耐碱性中起重要作用[30]。Mrp1复合物亚基同源性建模表明了两种可能的离子易位途径,推测其Lys299可能通过影响Mrp1A亚基中面向细胞质通道的稳定性和柔韧性发挥其耐碱性作用[30]。另外,CDF家族的假定转运蛋白Cgl1281可通过将有毒的Co2+外排来交换H+,提高C.glutamicum的耐碱性[31]。
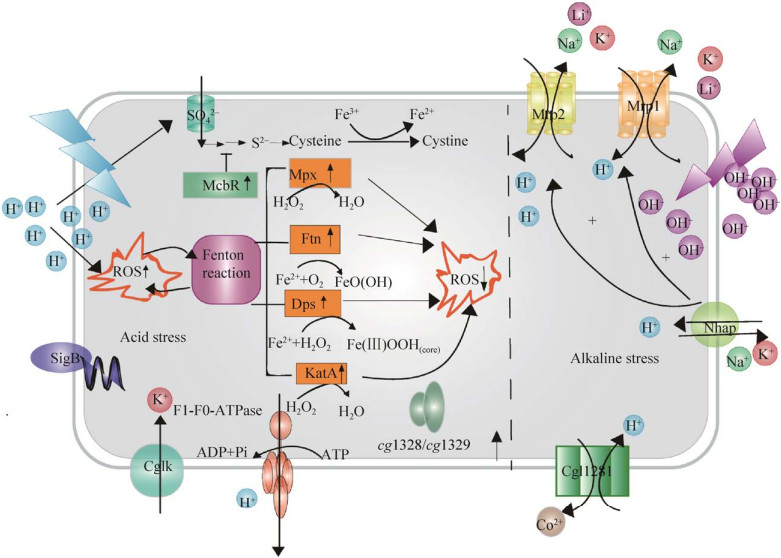 |
| 图 2 谷氨酸棒杆菌对pH波动胁迫的耐受机制 Fig. 2 Mechanisms of C.glutamicum responds to pH fluctuation. General acid adaptive mechanisms in C.glutamicum are shown on the left, and molecular strategies adopted by C.glutamicum for coping with alkaline stress are shown on the right. ROS: reactive oxygen species; McbR: sulfur metabolism regulator; SigB: sigma B factor; CglK: potassium channel protein; KatA: catalase; Dps: DNA protection during starvation protein; Ftn: ferritin-like protein; Copper chaperone system: cg1328, cg1329; Mpx: mycothiol peroxidase; Multiple resistance and pH adaptation antiporter: Mrp1, Mrp2; Nhap: Na+/H+ antiporter; Cgl1281: putative transporter of the cation difusion facilitator family. |
| 图选项 |
1.4 谷氨酸棒杆菌对氧化胁迫的耐受机制在工业发酵中,微生物菌株常面临氧化胁迫压力[32-33],尤其在发酵中后期活性氧对细胞生长和代谢具有较大威胁[32]。研究发现棒杆菌细胞在高溶氧发酵生产氨基酸和有机酸时会产生大量的ROS,显著影响其细胞生长和目标产物的代谢合成效率[34]。为应对ROS造成的损伤,保持良好的细胞氧化还原状态,研究发现谷氨酸棒杆菌已进化出较复杂的保护反应系统。
谷氨酸棒杆菌细胞可通过三道防线来适应ROS胁迫,如图 3所示:1) 生成分枝硫醇(Mycothiol,MSH):MSH作为非酶抗氧化剂,可以与ROS反应直接清除自由基;同时,MSH还可以作为麦硫酚过氧化物酶(Mpx)、蛋氨酸亚砜还原酶(MsrA)的辅助因子来保护细胞免受ROS损伤[35]。2) 合成ROS的解毒酶如超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、分枝硫醇二硫还原酶(Mtr) 等。当机体受到活性氧胁迫时,会诱导胞内CAT和SOD表达,两者协同清除胞内过多的H2O2与O2–。同时,Mtr催化氧化态分枝硫醇(MSSM) 为还原态分枝硫醇(MSH)[35]。3) 通过应激响应激活氧化传感器调控因子来维持氧化还原稳态。①氧化还原应答转录调控因子:氧化应激反应转录调控因子RosR[36]、醌氧化还原酶转录调控因子QorR[37]、有机过氧化物转录调控因子OhsR[38]、氧化还原敏感转录调控因子CosR[39]、有机过氧化物和抗生素敏感转录调控因子OsnR[40]会感知和响应不同种类的ROS并诱导相关酶基因表达来清除ROS。同时谷氨酸棒杆菌的OxyR作为氧化还原信号调控因子,应答胞内ROS水平后调节一系列的基因表达以抵抗氧化胁迫,包括katA、hemH、suf操纵子等23个基因的表达[41];② Sigma因子:C.glutamicum全局转录调控因子SigH对MSH再生的mca和mtr基因及与抗氧化有关的msrA和mpx基因有激活作用,而对硫醇过氧化酶基因prx有抑制作用,从而提高C.glutamicum的抗逆性和鲁棒性[35]。
 |
| 图 3 谷氨酸棒杆菌对氧化胁迫的耐受机制 Fig. 3 Mechanisms of C.glutamicum responds to oxidative stress. SigH: sigma H factor; Mpx: mycothiol peroxidase; MsrA: methionine sulfoxide reductase; Mca: branched thiol thiotransferamidase; MSH: mycothiol; MSSM: mycothiol disulfide; Mtr: mycothiol disulfde reductase; KatA: catalase; SOD: superoxide dismutase; CAT: catalase; Suf: suf cluster; transcription factors: QorR, RosR, OsnR, CosR, OhsR; MSH-R: mycothiol combined with environmental stimuli. |
| 图选项 |
1.5 谷氨酸棒杆菌对大规模培养中生物反应器不均一性的耐受机制生物过程的不均一性是工业规模和实验室规模生物过程之间的重要区别。随着生物反应器体积的增加,混合时间从几秒钟(实验室规模) 增加到几分钟(工业规模)[42-43]。工业规模生物反应器底部增加的静水压力提高了溶解气体的浓度,导致渗透压和pH的变化[44]。此外,其他生物反应器内部组件,例如喷射器、挡板和冷却盘管,可能会形成混合、传热和气液传质不佳的死区。为了降低生产成本,许多工艺使用的粗制原料很少或没有精制就被利用,从而将积聚在培养基中的杂质引入到抑制毒性水平[45-48]。
谷氨酸棒杆菌作为工业应用广泛的微生物菌株,其生产规模通常达到数百立方米的反应器工作量。由于混合时间增加、底物和氧气供应的不均匀、同质性的丧失会导致副产物快速流动,以及中间介质酸化并导致产物产量降低[49-51]。然而,谷氨酸棒杆菌对大规模培养的生物反应器不均一性具有鲁棒性。K??等[50]利用双室反应器研究C.glutamicum DM1933时发现,在底物限制、完全有氧和底物过量、限氧培养条件下,该菌具有抗振荡的代谢稳健性。Limberg等[52]研究发现产1, 5-二氨基戊烷的C.glutamicum DM1945Δact3- ldcCopt在短期耗氧和碳源过量的情况下,非氧气依赖性发酵NAD+的关键酶如L-乳酸脱氢酶和苹果酸脱氢酶的转录显著上调。上述研究表明,谷氨酸棒杆菌对工业规模生物反应器中发生的氧气和底物浓度变化有出色的抵抗力。
2 谷氨酸棒杆菌工业鲁棒性合成生物学元件开发2.1 功能基因元件在面对环境压力时,通过加强表达天然或外源功能结构基因是用于改善菌株耐受性的方法之一,见表 1。Xu等[25]利用C.glutamicum内源组成型天然启动子Psod和Pgap过表达了katA、dps、mcbR和cg1328耐酸性的功能模块,成功提高了谷氨酸棒杆菌菌株在酸性条件的生长速率和产量。何猛超[53]通过ARTP诱变技术高通量筛选得到了耐受pH 5.0及pH 11.0的C.glutamicum icgTAC02和icgTAI02。利用响应酸性环境的Pasr启动子及产酸基因pQO、aceE、idh和响应碱性环境的启动子Patp2及产碱基因glsA、gad在谷氨酸棒杆菌中构建了pH智能调节基因线路,实现了pH的自我智能调控。Man等[34]通过敲除C.glutamicum ATCC13032形成H2O2的黄素还原酶Frd181和Frd188并优化ATP供应,降低细胞ROS水平,改善细胞内微环境,提高了细胞生长得率,使得L-精氨酸产量提升到57.3 g/L。此外,提高细胞转运代谢产物的能力是提高菌株鲁棒性的另一手段[54],见表 2。Lubitz等[55]敲除C.glutamicum lysE基因并加强表达腐胺转运透性酶基因cgmA可以补偿由于缺乏LysE转运蛋白引起的生长缺陷,同时能够解除外源添加精氨酸二肽引发的细胞毒性。Krumbach等[56]使用CRISPR/Cas12a技术对谷氨酸棒杆菌基因组中的机械敏感通道蛋白MscCG进行定向突变并加强表达,增强了L-谷氨酸的分泌,减少了细胞内的代谢负担。Zhang等[57]利用来源大肠杆菌的5-氨基乙酰丙酸转运基因rthA和双组分系统HrrSA设计响应血红素的生物传感器Phmuo-rthA动态调节5-氨基乙酰丙酸外排,防止目标产物再次降解和积累。
表 1 功能基因元件增强谷氨酸棒杆菌鲁棒性举例Table 1 Examples of functional gene elements for enhancing robustness of C.glutamicum
| Strategy | Purpose | Gene source/host | Enhanced phenotype | References |
| Combined reengineering of missense mutant genes glmUE295K, otsAR328H | Adjust metabolic pathways | C.glutamicum | Heat tolerance | [58] |
| Heterologous expression of lysDH, proC | Accumulate compatible solute L-pipecolic acid | Silicibacter pomeroyi C.glutamicum | High osmotic pressure tolerance | [59] |
| Overexpression of katA, dps, mcbRandcg1328 | Reduce the level of intracellular ROS caused by acid stress and improve cell growth | C.glutamicum | Acid tolerance | [25] |
| Overexpression of pQO, aceE, idh and fine regulation of their expression levels | Produce acidic substances and neutralize alkaline | C.glutamicum | Alkaline tolerance | [53] |
| Overexpression of glsA, gad and fine regulation of their expression levels | Produce alkaline substances and neutralize acids | C.glutamicum | Acid tolerance | [53] |
| Overexpression of mpx | Eliminate ROS produced by acid stress | C.glutamicum | Acid tolerance | [60] |
| Overexpression of mshA | Eliminate ROS produced by acid stress | C.glutamicum | Acid tolerance | [61] |
| Overexpression of mtr | Eliminate ROS accumulated in cells | C.glutamicum | Oxidative stress | [62] |
表选项
表 2 分泌转运蛋白工程增强谷氨酸棒杆菌鲁棒性举例Table 2 Examples of exporter engineering for enhancing robustness of C.glutamicum
| Exporter | Export substance | Method | Enhanced phenotype and representative effect | Reference |
| CgmA | L-arginine | Deletion of cgmR and lysE; Overexpression of cgmA Overexpression of brnFE | Improve cell growth and relieve cytotoxicity caused by exogenous addition of arginine dipeptide | [55] |
| BrnFE | L-valine | Improve the transport capacity of valine and make the output reach 461.4 mmol/L | [63] | |
| SucE | Succinic acid | Overexpression of sucE | Increase succinic acid production and reduce intracellular accumulation | [64] |
| CgynfM | Succinic acid | Overexpression of cgynfM | Reduce by-product formation, increased aerobic succinate production by 107 mmol/L | [65] |
| MscCG2 | L-glutamate | Overexpression and directed mutation of mscCG2 | Enhance secretion of L-glutamic acid, reduce intracellular accumulation and metabolic burden, and increase yield to 130 mmol/L | [56] |
| Cg2893 | 1, 5-diaminopentane | Deletion of lysE and cg2894; Overexpression of cgmA | Improve growth, increase yield by 20% and reduce by-products N-acetyl-diaminopentane by 75% | [66] |
| RthA | 5-aminolevulinic acid | Heterologous expression of rthA | Construct Phmuo-rthA biosensor to dynamically adjust 5-aminolevulinic acid efflux to prevent the target product from degrading and accumulating again | [57] |
表选项
2.2 转录调控因子原核生物具有控制基因转录的金字塔形分级调节网络,可以快速响应短暂的环境变化例如pH、温度、营养和渗透压,并优化其代谢以适应新环境。近年来,研究人员通过挖掘天然转录调控因子、人工设计转录因子和引入外源全局调节剂IrrE等显著提高了细胞对不同压力的耐受性[13]。Kim等[67]使用绿色荧光蛋白作为报告蛋白筛选出SigB依赖性启动子Pcg3141并对其启动子进行改造,使其在C.glutamicum的指数生长期与稳定期之间的过渡期被激活。另外,基于转录调控因子构建响应代谢终产物或有毒中间体的生物传感器可以平衡代谢路径通量、减轻代谢负担、避免不需要的中间体的积累,从而提高菌株的抗逆性。Xu等[68]设计基于FapR的丙二酰辅酶A调控因子型生物传感器(TF-Biosensor) 可用于负调控上游乙酰辅酶A羧化酶ACC和正调控下游脂肪酸生物合成操纵子,减少脂肪酸合成途径中有毒中间体丙二酰辅酶A的生成,使脂肪酸效价提高2.1倍。Zhang等[69]设计了脂酰基辅酶ATF生物传感器FadR,通过感应脂肪酸/脂肪酰基辅酶A的水平来平衡乙醇和脂肪酸的生物合成途径,减少了乙醇积累,防止细胞脂肪酸消耗,从而使脂肪酸乙酯产量增加3倍。谷氨酸棒杆菌目前已表征功能参与各种应激反应的转录调控因子很多,见表 3。改造转录调控因子元件或利用转录调控因子设计代谢物传感单元全局扰动代谢反应及动态控制基因表达实现代谢途径平衡是未来提高谷氨酸棒杆菌鲁棒性的有效方向。
表 3 谷氨酸棒杆菌中参与应激反应的转录因子Table 3 Examples of transcription factors of C.glutamicum involved in stress response
| Transcription Factor | Coding gene | Stress response | Reference |
| SigH | cg0876 | Oxidative and Heat stress | [74] |
| SigM | cg3420 | Oxidative and Heat stress | [75] |
| SigE | cg1271 | Heat and organic solvent stress | [76] |
| OxyR | cg2109 | Oxidative stress | [41] |
| RosR | cg1324 | Oxidative stress | [36] |
| QorR | cg1552 | Oxidative stress | [37] |
| CosR | cg3001 | Oxidative stress | [39] |
| OsnR | cg3230 | Oxidative stress | [40] |
| OhsR | cg0039 | Oxidative stress | [38] |
表选项
2.3 Small RNASmall RNA (sRNA) 是细菌体内广泛存在的一类非编码RNA,在转录水平通过与mRNA相互作用快速调控目标基因表达,在环境压力响应过程中发挥关键的调控作用。与需要蛋白质合成的转录因子相比,在途径工程中使用sRNA可赋予相对较低的代谢负荷。因此,sRNA已经作为重要工具用于提高细菌抗逆性能[70]。例如Na等[71]开发了一种系统来设计温度诱导的合成sRNA,可以根据温度变化改变sRNA的结合能力,微调基因表达。Gaida等[72]通过过表达E.coli几种sRNA (DsrA、RprA和ArcZ),在活跃的细胞生长过程中,对酸性环境的耐受性提高了8 500倍,并为细胞提供了抵抗羧酸和氧化应激的保护作用。
目前针对C.glutamicum中sRNA响应环境压力和调控的机制研究较少。Mentz等[73]利用RNA测序首次预测C.glutamicum ATCC13032基因组中可能编码262个sRNA,并通过实验发现cgb-00105的转录水平会在高温条件下显著下调。Pahlke等[77]研究发现C.glutamicum在细胞分裂期间若缺失高度保守的6CRNA (cgb-03605) 会对紫外损伤和丝裂霉素更加敏感,因此确定6CRNA参与调控DNA损伤修复。Zemanová等[78]发现C.glutamicum cg1935上游存在一个高温诱导的sRNA-ArnA。而最近Sun等[79]针对谷氨酸棒杆菌开发了基于合成的sRNA的基因敲弱策略;他们基于E.coli MicC人工合成sRNA及Hfq引入C.glutamicum,发现报告基因表达及mRNA水平均大幅下降,表明sRNA-Hfp复合体可以在C.glutamicum中与靶基因结合,并借助内源RNA酶将mRNA降解。将合成的sRNA系统用来分别降低丙酮酸激酶(pyk)、乳酸脱氢酶(ldhA) 和2-氧戊二酸脱氢酶复合物E1亚基(odhA) 基因表达水平,从而使谷氨酸滴度和产量提高了近3倍。总之,sRNA可以精确、灵活和动态地调控靶基因的表达,可以用作提高菌株对环境胁迫的抗逆性元件。
2.4 适应性进化策略挖掘合成生物学抗逆元件基因相互作用网络和调控系统的复杂性使得单纯利用代谢工程获得具有耐受性的菌株具有挑战性。对于遗传基础复杂难以确定的性状改造,自适应实验室进化(ALE) 作为在耐受性工程中广泛使用的高效工具,已用于创建具有优良性能的谷氨酸棒杆菌株,如表 4所示。对利用ALE策略获得的生产菌株进行基因组测序和组学分析可以获得生物途径有关酶特性或途径调控的新信息。再通过反向代谢工程等手段,可以进一步稳定或增强这种特性以达到获得优良性状的目的。
表 4 适应性进化策略挖掘抗逆元件鲁增强谷氨酸棒杆菌鲁棒性举例Table 4 Examples of adaptive laboratory evolution strategies to discovering stress-resistant elements for enhancing robustness of C.glutamicum
| ALE target | Phenotype improvement | Sequencing/transcriptomics | Proven causal mutations | References |
| Increased growth at high temperatures | Improve growth at suboptimal temperatures and Adapt to the growth temperature broadened to 41.5 ℃ | Deletion of two genomic regions, 295 total point mutations | Missense mutation in glmU and otsA | [58] |
| Increased to lerance to inhibitors in corn stover hydrolysate | Improve cell growth and Increased degradation rate of several inhibitors | Seven mutations in evolution strains | Not determined | [80] |
| Increased tolerance to methanol | Improve cell growth rate and increase methanol utilization | All the three evolved strains harbored 10 mutations | Mutation in Cgl2857, Cgl1063(MetY) and Cgl0833 | [81] |
| Increased tolerance to H2O2 | Developed the ability to grow under stress of 10 mmol/L H2O2 | No sequencing performed; transcriptome analysis using RNA-seq | Not determined | [82] |
表选项
Oide等[58]利用ALE挖掘出来的功能基因元件glmUE295K (双功能N-乙酰氨基葡萄糖-1-磷酸尿酰转移酶/氨基葡萄糖-1-磷酸乙酰转移酶) 和otsAR328H (海藻糖-6-磷酸合成酶),成功将C.glutamicum GLY3的适应生长温度拓宽,比生长速率提高。Wang等[80]使用ALE策略提高了C.glutamicum S9114对玉米秸秆水解产物中存在的抑制剂的耐受性。转录分析表明,在进化菌株中可能由于葡萄糖-PTS转运系统和磷酸戊糖途径显著上调,为醛类抑制剂还原转化提供充足的NAD(P)H供应,增强了抑制剂的耐受性。中国科学院天津工业生物技术研究所孙际宾和郑平[81]实验室通过耐受性工程和ALE策略改善了细胞对甲醇的耐受性从而获得具有较高甲醇生物转化能力的谷氨酸棒杆菌。突变体菌株MX14在高浓度甲醇存在下显示出明显的生长优势。其比较转录组学数据显示基因Cg10653、Cgl2857、Cgl0833突变可以提高细胞对高浓度甲醇的耐受性。上述案例说明,利用ALE策略挖掘功能基因元件,构建抗逆基因线路对改造谷氨酸棒杆菌底盘细胞工业鲁棒性具有重要作用。
3 利用合成生物学设计基因线路提高谷氨酸棒杆菌底盘细胞工业鲁棒性在合成生物学中,面对胞内外环境变化,利用不同调控元件如启动子、SD序列与抗逆功能基因和调节基因等构建基因线路并对线路模块进行动态调节是提高系统鲁棒性的有效策略[83-84]。Tung等[85]构建了具有基因整合和表达能力的生物传感器MRX1-roGFP2,可在氧化应激和生长过程中,实时跟踪C.glutamicum全基因组突变对MSH氧化还原电位变化的影响以及监测发酵过程中细胞的氧化还原状态。Binder等[86]开发了一种光控开关,可控制信号物质NP-photocaged IPTG (异丙基-β-D-硫代半乳糖苷衍生物) 的光解速度,进而控制Plac启动子驱动朱栾倍半萜合酶基因的表达水平,从而降低了终产物倍半萜烯(+)-瓦伦烯对菌体生长的影响,由此增强C.glutamicum底盘细胞鲁棒性并提高了目标产物的产量。Kobayashi等[87]使用氧气作为自动诱导开关,利用乳酸脱氢酶基因idhA厌氧诱导型启动子代替葡萄糖-6-磷酸异构酶基因pgi启动子,实现有氧条件下将83%的碳通量导入戊糖磷酸途径,从而使生成1-5-二氨基戊烷产量、产率和生产速率分别提升4.6倍、4.4倍和2.6倍。Tan等[88]利用生物传感器Lrp-PbrnFE对4-羟基异亮氨酸生物合成途径关键基因odhI (α-酮戊二酸脱氢酶复合物抑制物)、vgb (透明颤菌血红蛋白VHb)、ido (异亮氨酸双加氧酶)的表达进行动态控制,协同调节底物α-酮戊二酸、O2和异亮氨酸供给,解决了底物之一氧气过量供给引起活性氧损伤细胞的问题,因此增强了C.glutamicum底盘细胞鲁棒性。Zhang等[57]结合静态代谢工程(弱启动子)、动态代谢工程(温度敏感质粒pFST)、自诱导代谢工程(生长相关启动子Pcp_2836) 和响应血红素的生物传感器(Phmuo-rthA) 构建了动态调节5-氨基乙酰丙酸合成及外排的基因线路,最终5-氨基乙酰丙酸产量达到3.61 g/L。
4 总结与展望近几十年来,通过合成生物学及相关技术对谷氨酸棒杆菌细胞工厂的稳健性进行工程设计,重塑了细胞对压力的响应,并优化了目标代谢通量的动态控制,达到降低其受环境胁迫影响的目的。然而,在许多情况下,抗逆性是一种复杂的多基因表型,提高谷氨酸棒杆菌鲁棒性依旧是个重大挑战。因此,为了进一步开发和改进谷氨酸棒杆菌底盘细胞的鲁棒性和适配,未来的工作将集中在以下方面:1) 借助转录组学、合成生物学、生物信息学、分子生物学等新技术,挖掘响应溶氧、温度、pH、渗透压变化的转录调控因子、启动子、敏感型蛋白和sRNA,分析动态响应环境变化的分子机制,建立谷氨酸棒杆菌抗逆调控网络模型,丰富对谷氨酸棒杆菌抗逆调控机制的认识。2) 挖掘并构建启动子、RBS文库并优化启动子和RBS文库或筛选天然生物传感器、利用计算机人工设计生物传感器、转录因子来对合成途径上基因表达强度动态调节,设计稳健、灵敏、高效的基因线路,以适应不断变化的条件,进而提高谷氨酸棒杆菌鲁棒性。如图 4所示,在外界压力环境下,通过改造或进化sigma因子来直接或间接地改变转录调节网络,增强工业相关性的微生物胁迫耐受性;此外,可以利用转录调控因子和外源转运蛋白构建生物传感器来感应细胞内有毒代谢物或代谢产物的浓度,减少有毒代谢物的积累并加快代谢产物的排出,从而降低有毒物质对细胞的损伤和减少细胞的代谢负担;或者对内源转运蛋白进行改造,提高对转运底物的特异性和转运能力,高效分泌转运胞内产物;另外,使用不同强度、类型的启动子、构建调控元件并设计基因线路精确调节物质流及能量流解决细胞生长阻滞和毒性中间体积累等问题,从而提高谷氨酸棒杆菌底盘细胞鲁棒性。总之,未来依然需要借助于系统生物学等分析手段,全面对谷氨酸棒杆菌功能基因组分析,继续挖掘提高鲁棒性相关功能元件,最终实现系统、全局的改造以达到可使用的细胞工厂高产量、高速率、高产率和高纯度的目标。
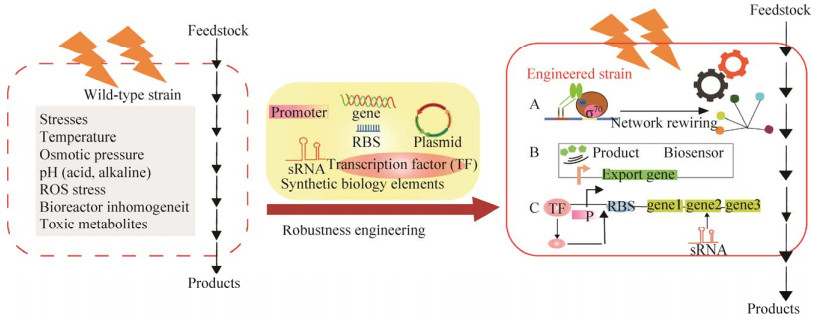 |
| 图 4 合成生物学增强谷氨酸棒杆菌工业鲁棒性中的应用 Fig. 4 Application of synthetic biology approaches for enhancing the industrial robustness of C. glutamicum. A: global transcription factor engineering makes the cell reach a fitness state to tolerate harsh conditions; B: using transcription factors to construct biosensors to dynamically control product efflux; C: stress resistance elements to construct gene circuits to enhance the robustness of chassis cells. |
| 图选项 |
参考文献
| [1] | Yang YP, Lin YH, Li LY, et al. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metabol Eng, 2015, 29: 217-226. DOI:10.1016/j.ymben.2015.03.018 |
| [2] | Humphreys CM, Minton NP. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas. Curr Opin Biotechnol, 2018, 50: 174-181. DOI:10.1016/j.copbio.2017.12.023 |
| [3] | Wang J, Zhang RH, Zhang Y, et al. Developing a pyruvate-driven metabolic scenario for growth-coupled microbial production. Metab Eng, 2019, 55: 191-200. DOI:10.1016/j.ymben.2019.07.011 |
| [4] | Longwell CK, Labanieh L, Cochran JR. High-throughput screening technologies for enzyme engineering. Curr Opin Biotechnol, 2017, 48: 196-202. DOI:10.1016/j.copbio.2017.05.012 |
| [5] | Wu WJ, Zhang Y, Liu DH, et al. Efficient mining of natural NADH-utilizing dehydrogenases enables systematic cofactor engineering of lysine synthesis pathway of Corynebacterium glutamicum. Metab Eng, 2019, 52: 77-86. DOI:10.1016/j.ymben.2018.11.006 |
| [6] | Wang J, Shen XL, Jain R, et al. Establishing a novel biosynthetic pathway for the production of 3, 4-dihydroxybutyric acid from xylose in Escherichia coli. Metabol Eng, 2017, 41: 39-45. DOI:10.1016/j.ymben.2017.03.003 |
| [7] | Gong ZW, Nielsen J, Zhou YJ. Engineering robustness of microbial cell factories. Biotechnol J, 2017, 12(10): 1700014. DOI:10.1002/biot.201700014 |
| [8] | Yang YP, Lin YH, Wang J, et al. Sensor-regulator and RNAi based bifunctional dynamic control network for engineered microbial synthesis. Nat Commun, 2018, 9(1): 3043. DOI:10.1038/s41467-018-05466-0 |
| [9] | Choi KR, Jang WD, Yang D, et al. Systems metabolic engineering strategies: integrating systems and synthetic biology with metabolic engineering. Trends Biotechnol, 2019, 37(8): 817-837. DOI:10.1016/j.tibtech.2019.01.003 |
| [10] | Zhang Y, Cai JY, Shang XL, et al. A new genome-scale metabolic model of Corynebacterium glutamicum and its application. Biotechnol Biofuels, 2017, 10: 169. DOI:10.1186/s13068-017-0856-3 |
| [11] | Kogure T, Inui M. Recent advances in metabolic engineering of Corynebacterium glutamicum for bioproduction of value-added aromatic chemicals and natural products. Appl Microbiol Biotechnol, 2018, 102(20): 8685-8705. DOI:10.1007/s00253-018-9289-6 |
| [12] | Lee MJ, Kim P. Recombinant protein expression system in Corynebacterium glutamicum and its application. Front Microbiol, 2018, 9: 2523. DOI:10.3389/fmicb.2018.02523 |
| [13] | Lin ZL, Zhang Y, Wang JQ. Engineering of transcriptional regulators enhances microbial stress tolerance. Biotechnol Adv, 2013, 31(6): 986-991. DOI:10.1016/j.biotechadv.2013.02.010 |
| [14] | Walker GM, Dijck PV. Physiological and molecular responses of yeasts to the environment//Querol A, Fleet G. Yeasts in Food and Beverages. Berlin: Springer, 2006: 111-152. |
| [15] | Barreiro C, González-Lavado E, Brand S, et al. Heat shock proteome analysis of wild-type Corynebacterium glutamicum ATCC 13032 and a spontaneous mutant lacking GroEL1, a dispensable chaperone. J Bacteriol, 2005, 187(3): 884-889. DOI:10.1128/JB.187.3.884-889.2005 |
| [16] | Kallscheuer N, Bott M, Ooyen JV, et al. Single-domain peptidyl-prolyl cis/trans isomerase FkpA from Corynebacterium glutamicum improves the biomass yield at increased growth temperatures. Appl Environ Microbiol, 2015, 81(22): 7839-7850. DOI:10.1128/AEM.02113-15 |
| [17] | Ventura M, Canchaya C, Zhang ZD, et al. How high G+C Gram-positive bacteria and in particular bifidobacteria cope with heat stress: protein players and regulators. FEMS Microbiol Rev, 2006, 30(5): 734-759. DOI:10.1111/j.1574-6976.2006.00031.x |
| [18] | Matsutani M, Nantapong N, Murata R, et al. Complete genome sequencing of newly isolated thermotolerant Corynebacterium glutamicum N24 provides a new insights into its thermotolerant phenotype. J Biotechnol, 2017, 247: 29-33. DOI:10.1016/j.jbiotec.2017.02.025 |
| [19] | Pérez-García F, Brito LF, Wendisch VF. Function of L-pipecolic acid as compatible solute in Corynebacterium glutamicum as basis for its production under hyperosmolar conditions. Front Microbiol, 2019, 10: 340. DOI:10.3389/fmicb.2019.00340 |
| [20] | M?ker N, Kr?mer J, Unden G, et al. In vitro analysis of the two-component system MtrB-MtrA from Corynebacterium glutamicum. J Bacteriol, 2007, 189(9): 3645-3649. DOI:10.1128/JB.01920-06 |
| [21] | Kr?mer R. Osmosensing and osmosignaling in Corynebacterium glutamicum. Amino Acids, 2009, 37(3): 487-497. DOI:10.1007/s00726-009-0271-6 |
| [22] | Kawasaki H, Martinac B. Mechanosensitive channels of Corynebacterium glutamicum functioning as exporters of L-glutamate and other valuable metabolites. Curr Opin Chem Biol, 2020, 59: 77-83. DOI:10.1016/j.cbpa.2020.05.005 |
| [23] | B?rngen K, Battle AR, M?ker N, et al. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim Biophys Acta, 2010, 1798(11): 2141-2149. DOI:10.1016/j.bbamem.2010.06.022 |
| [24] | Follmann M, Ochrombel I, Kr?mer R, et al. Functional genomics of pH homeostasis in Corynebacterium glutamicum revealed novel links between pH response, oxidative stress, iron homeostasis and methionine synthesis. BMC Genomics, 2009, 10: 621. DOI:10.1186/1471-2164-10-621 |
| [25] | Xu N, Lv HF, Wei L, et al. Impaired oxidative stress and sulfur assimilation contribute to acid tolerance of Corynebacterium glutamicum. Appl Microbiol Biotechnol, 2019, 103(4): 1877-1891. DOI:10.1007/s00253-018-09585-y |
| [26] | Michel A, Koch-Koerfges A, Krumbach K, et al. Anaerobic growth of Corynebacterium glutamicum via mixed-acid fermentation. Appl Environ Microbiol, 2015, 81(21): 7496-7508. DOI:10.1128/AEM.02413-15 |
| [27] | Lund P, Tramonti A, De Biase D. Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev, 2014, 38(6): 1091-1125. DOI:10.1111/1574-6976.12076 |
| [28] | Follmann M, Becker M, Ochrombel I, et al. Potassium transport in corynebacterium glutamicum is facilitated by the putative channel protein CglK, which is essential for pH homeostasis and growth at acidic pH. J Bacteriol, 2009, 191(9): 2944-2952. DOI:10.1128/JB.00074-09 |
| [29] | Jakob K, Satorhelyi P, Lange C, et al. Gene expression analysis of Corynebacterium glutamicum subjected to long-term lactic acid adaptation. J Bacteriol, 2007, 189(15): 5582-5590. DOI:10.1128/JB.00082-07 |
| [30] | Xu N, Zheng YY, Wang XC, et al. The lysine 299 residue endows the multisubunit Mrp1 antiporter with dominant roles in Na+ resistance and pH homeostasis in Corynebacterium glutamicum. Appl Environ Microbiol, 2018, 84(10): e00110-18. |
| [31] | Guo J, Ma ZP, Gao JS, et al. Recent advances of pH homeostasis mechanisms in Corynebacterium glutamicum. World J Microbiol Biotechnol, 2019, 35(12): 192. DOI:10.1007/s11274-019-2770-2 |
| [32] | Zhang MM, Wang YJ, Ang EL, et al. Engineering microbial hosts for production of bacterial natural products. Nat Prod Rep, 2016, 33(8): 963-987. DOI:10.1039/C6NP00017G |
| [33] | Ezraty B, Gennaris A, Barras F, et al. Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol, 2017, 15(7): 385-396. DOI:10.1038/nrmicro.2017.26 |
| [34] | Man ZW, Rao ZM, Xu MJ, et al. Improvement of the intracellular environment for enhancing L-arginine production of Corynebacterium glutamicum by inactivation of H2O2-forming flavin reductases and optimization of ATP supply. Metab Eng, 2016, 38: 310-321. DOI:10.1016/j.ymben.2016.07.009 |
| [35] | 司美茹. 谷氨酸棒杆菌中分枝硫醇抗环境胁迫的作用机制研究[D]. 杨凌: 西北农林科技大学, 2014. Si MR. Mechanisms of mycothiol in resistance to multiple environmental stresses in Corynebacterium glutamicum[D]. Yangling: Northwest Agriculture and Forestry University, 2014. |
| [36] | Bussmann M, Baumgart M, Bott M. RosR (Cg1324), a hydrogen peroxide-sensitive MarR-type transcriptional regulator of Corynebacterium glutamicum. J Biol Chem, 2010, 285(38): 29305-29318. DOI:10.1074/jbc.M110.156372 |
| [37] | Ehira S, Ogino H, Teramoto H, et al. Regulation of quinone oxidoreductase by the redox-sensing transcriptional regulator QorR in Corynebacterium glutamicum. J Biol Chem, 2009, 284(25): 16736-16742. DOI:10.1074/jbc.M109.009027 |
| [38] | Si MR, Su T, Chen C, et al. OhsR acts as an organic peroxide-sensing transcriptional activator using an S-mycothiolation mechanism in Corynebacterium glutamicum. Microb Cell Fact, 2018, 17(1): 200. DOI:10.1186/s12934-018-1048-y |
| [39] | Si M, Chen C, Su T, et al. CosR is an oxidative stress sensing a MarR-type transcriptional repressor in Corynebacterium glutamicum. Biochem J, 2018, 475(24): 3979-3995. DOI:10.1042/BCJ20180677 |
| [40] | Jeong H, Kim Y, Lee HS. The osnR gene of Corynebacterium glutamicum plays a negative regulatory role in oxidative stress responses. J Ind Microbiol Biotechnol, 2019, 46(2): 241-248. DOI:10.1007/s10295-018-02126-6 |
| [41] | Milse J, Petri K, Rückert C, et al. Transcriptional response of Corynebacterium glutamicum ATCC 13032 to hydrogen peroxide stress and characterization of the OxyR regulon. J Biotechnol, 2014, 190: 40-54. DOI:10.1016/j.jbiotec.2014.07.452 |
| [42] | Humphrey A. Shake flask to fermentor: what have we learned?. Biotechnol Pro, 1998, 14: 3-7. |
| [43] | Schmidt FR. Optimization and scale up of industrial fermentation processes. Appl Microbiol Biotechnol, 2005, 68(4): 425-435. DOI:10.1007/s00253-005-0003-0 |
| [44] | Kumar D, Murthy GS. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol Biofuels, 2011, 4: 27. DOI:10.1186/1754-6834-4-27 |
| [45] | Deparis Q, Claes A, Foulquié-Moreno MR, et al. Engineering tolerance to industrially relevant stress factors in yeast cell factories. FEMS Yeast Res, 2017, 17(4): fox03. |
| [46] | Fischer CR, Klein-Marcuschamer D, Stephanopoulos G. Selection and optimization of microbial hosts for biofuels production. Metab Eng, 2008, 10(6): 295-304. DOI:10.1016/j.ymben.2008.06.009 |
| [47] | Mukhopadhyay A. Tolerance engineering in bacteria for the production of advanced biofuels and chemicals. Trends Microbiol, 2015, 23(8): 498-508. DOI:10.1016/j.tim.2015.04.008 |
| [48] | Wehrs M, Tanjore D, Eng T, et al. Engineering robust production microbes for large-scale cultivation. Trends Microbiol, 2019, 27(6): 524-537. DOI:10.1016/j.tim.2019.01.006 |
| [49] | K?? F, Junne S, Neubauer P, et al. Process inhomogeneity leads to rapid side product turnover in cultivation of Corynebacterium glutamicum. Microb Cell Fact, 2014, 13: 6. DOI:10.1186/1475-2859-13-6 |
| [50] | K?? F, Hariskos I, Michel A, et al. Assessment of robustness against dissolved oxygen/substrate oscillations for C.glutamicum DM1933 in two-compartment bioreactor. Bioprocess Biosyst Eng, 2014, 37(6): 1151-1162. DOI:10.1007/s00449-013-1086-0 |
| [51] | Olughu W, Nienow A, Hewitt C, et al. Scale-down studies for the scale-up of a recombinant Corynebacterium glutamicum fed-batch fermentation: loss of homogeneity leads to lower levels of cadaverine production. J Chem Technol Biotechnol, 2020, 95(3): 675-685. DOI:10.1002/jctb.6248 |
| [52] | Limberg MH, Schulte J, Aryani T, et al. Metabolic profile of 1, 5-diaminopentane producing Corynebacterium glutamicum under scale-down conditions: Blueprint for robustness to bioreactor inhomogeneities. Biotechnol Bioeng, 2016, 114(3): 560-575. |
| [53] | 何猛超. 基于ARTP诱变的耐酸/碱谷氨酸棒杆菌的筛选及其pH智能调节系统的构建[D]. 延安: 延安大学, 2020. He MC. Screening of Acid/alkali-tolerance Corynebacterium Glutamicum based on ARTP mutagenesis and construction of ph intelligent adjustment system[D]. Yan'an: Yan'an University, 2020 (in Chinese). |
| [54] | 张晓梅, 高宇洁, 杨玲, 等. 谷氨酸棒杆菌中氨基酸分泌转运蛋白及其代谢改造研究进展. 生物工程学报, 2020, 36(11): 2250-2259. Zhang XM, Gao YJ, Yang L, et al. Advances in amino acid exporters and its metabolic modification of Corynebacterium glutamicum. Chin J Biotech, 2020, 36(11): 2250-2259 (in Chinese). |
| [55] | Lubitz D, Jorge JMP, Pérez-García F, et al. Roles of export genes cgmA and lysE for the production of L-arginine and L-citrulline by Corynebacterium glutamicum. Appl Microbiol Biotechnol, 2016, 100(19): 8465-8474. DOI:10.1007/s00253-016-7695-1 |
| [56] | Krumbach K, Sonntag CK, Eggeling L, et al. CRISPR/Cas12a mediated genome editing to introduce amino acid substitutions into the mechanosensitive channel MscCG of Corynebacterium glutamicum. ACS Synth Biol, 2019, 8(12): 2726-2734. DOI:10.1021/acssynbio.9b00361 |
| [57] | Zhang CL, Li YJ, Zhu FZ, et al. Metabolic engineering of an auto-regulated Corynebacterium glutamicum chassis for biosynthesis of 5-aminolevulinic acid. Bioresour Technol, 2020, 318: 124064. DOI:10.1016/j.biortech.2020.124064 |
| [58] | Oide S, Gunji W, Moteki Y, et al. Thermal and solvent stress cross-tolerance conferred to Corynebacterium glutamicum by adaptive laboratory evolution. Appl Environ Microbiol, 2015, 81(7): 2284-2298. DOI:10.1128/AEM.03973-14 |
| [59] | Pérez-García F, Peters-Wendisch P, Wendisch VF. Engineering Corynebacterium glutamicum for fast production of L-lysine and L-pipecolic acid. Appl Microbiol Biotechnol, 2016, 100(18): 8075-8090. DOI:10.1007/s00253-016-7682-6 |
| [60] | Wang TT, Gao F, Kang YW, et al. Mycothiol peroxidase MPx protects Corynebacterium glutamicum against acid stress by scavenging ROS. Biotechnol Lett, 2016, 38(7): 1221-1228. DOI:10.1007/s10529-016-2099-y |
| [61] | Liu YB, Chen C, Chaudhry MT, et al. Enhancing Corynebacterium glutamicum robustness by over-expressing a gene, mshA, for mycothiol glycosyltransferase. Biotechnol Lett, 2014, 36(7): 1453-1459. DOI:10.1007/s10529-014-1501-x |
| [62] | Liu YB, Long MX, Yin YJ, et al. Physiological roles of mycothiol in detoxification and tolerance to multiple poisonous chemicals in Corynebacterium glutamicum. Arch Microbiol, 2013, 195(6): 419-429. DOI:10.1007/s00203-013-0889-3 |
| [63] | 张海灵, 李颜颜, 王小元. 代谢工程改造谷氨酸棒状杆菌合成及分泌途径生产L-缬氨酸. 生物工程学报, 2018, 34(10): 1606-1619. Zhang HL, Li YY, Wang XY. Metabolic engineering of L-valine synthesis and secretory pathways in Corynebacterium glutamicum for higher production. Chin J Biotechnol, 2018, 34(10): 1606-1619 (in Chinese). |
| [64] | Huhn S, Jolkver E, Kr?mer R, et al. Identification of the membrane protein SucE and its role in succinate transport in Corynebacterium glutamicum. Appl Microbiol Biotechnol, 2011, 89(2): 327-335. DOI:10.1007/s00253-010-2855-1 |
| [65] | Fukui K, Nanatani K, Nakayama M, et al. Corynebacterium glutamicum CgynfM encodes a dicarboxylate transporter applicable to succinate production. J Biosci Bioeng, 2019, 127(4): 465-471. DOI:10.1016/j.jbiosc.2018.10.004 |
| [66] | Kind S, Kreye S, Wittmann C. Metabolic engineering of cellular transport for overproduction of the platform chemical 1, 5-diaminopentane in Corynebacterium glutamicum. Metab Eng, 2011, 13(5): 617-627. DOI:10.1016/j.ymben.2011.07.006 |
| [67] | Kim MJ, Yim SS, Choi JW, et al. Development of a potential stationary-phase specific gene expression system by engineering of SigB-dependent cg3141 promoter in Corynebacterium glutamicum. Appl Microbiol Biotechnol, 2016, 100(10): 4473-4483. DOI:10.1007/s00253-016-7297-y |
| [68] | Xu P, Li LY, Zhang FM, et al. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci USA, 2014, 111(31): 11299-11304. DOI:10.1073/pnas.1406401111 |
| [69] | Zhang FZ, Carothers JM, Keasling JD. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol, 2012, 30(4): 354-359. DOI:10.1038/nbt.2149 |
| [70] | Wang ZQ, Cirino PC. New and improved tools and methods for enhanced biosynthesis of natural products in microorganisms. Curr Opin Biotechnol, 2016, 42: 159-168. DOI:10.1016/j.copbio.2016.05.003 |
| [71] | Na D, Yoo SM, Chung H, et al. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat Biotechnol, 2013, 31(2): 170-174. DOI:10.1038/nbt.2461 |
| [72] | Gaida SM, Al-Hinai MA, Indurthi DC, et al. Synthetic tolerance: three noncoding small RNAs, DsrA, ArcZ and RprA, acting supra-additively against acid stress. Nucleic Acids Res, 2013, 41(18): 8726-8737. DOI:10.1093/nar/gkt651 |
| [73] | Mentz A, Neshat A, Pfeifer-Sancar K, et al. Comprehensive discovery and characterization of small RNAs in Corynebacterium glutamicum ATCC 13032. BMC Genomics, 2013, 14(1): 714. DOI:10.1186/1471-2164-14-714 |
| [74] | Dostálová H, Busche T, Holátko J, et al. Overlap of promoter recognition specificity of stress response sigma factors SigD and SigH in Corynebacterium glutamicum ATCC 13032. Front Microbiol, 2018, 9: 3287. |
| [75] | Nakunst D, Larisch C, Hüser AT, et al. The extracytoplasmic function-type sigma factor sigM of Corynebacterium glutamicum ATCC 13032 is involved in transcription of disulfide stress-related genes. J Bacteriol, 2007, 189(13): 4696-4707. DOI:10.1128/JB.00382-07 |
| [76] | 刘秀霞, 高雄, 白仲虎. 谷氨酸棒杆菌中选择性σ因子的研究进展. 微生物学通报, 2016, 43(10): 2261-2268. Liu XX, Gao X, Bai ZH. Advances in the alternative σ factors of Corynebacterium glutamicum. Microbiol China, 2016, 43(10): 2261-2268 (in Chinese). |
| [77] | Pahlke J, Dostálová H, Holátko J, et al. The small 6C RNA of Corynebacterium glutamicum is involved in the SOS response. RNA Biol, 2016, 13(9): 848-860. DOI:10.1080/15476286.2016.1205776 |
| [78] | Zemanová M, Kade?ábková P, Pátek M, et al. Chromosomally encoded small antisense RNA in Corynebacterium glutamicum. FEMS Microbiol Lett, 2008, 279(2): 195-201. DOI:10.1111/j.1574-6968.2007.01024.x |
| [79] | Sun DH, Chen JZ, Wang Y, et al. Metabolic engineering of Corynebacterium glutamicum by synthetic small regulatory RNAs. J Ind Microbiol Biotechnol, 2019, 46(2): 203-208. DOI:10.1007/s10295-018-02128-4 |
| [80] | Wang X, Khushk I, Xiao YQ, et al. Tolerance improvement of Corynebacterium glutamicum on lignocellulose derived inhibitors by adaptive evolution. Appl Microbiol Biotechnol, 2018, 102(1): 377-388. DOI:10.1007/s00253-017-8627-4 |
| [81] | Wang Y, Fan LW, Tuyishime P, et al. Adaptive laboratory evolution enhances methanol tolerance and conversion in engineered Corynebacterium glutamicum. Commun Biol, 2020, 3(1): 217. DOI:10.1038/s42003-020-0954-9 |
| [82] | Lee JY, Seo J, Kim ES, et al. Adaptive evolution of Corynebacterium glutamicum resistant to oxidative stress and its global gene expression profiling. Biotechnol Lett, 2013, 35(5): 709-717. DOI:10.1007/s10529-012-1135-9 |
| [83] | 秦磊, 俞杰, 宁小钰, 等. 合成生物系统构建与绿色生物"智"造. 化工学报, 2020, 71(9): 3979-3994. Qin L, Yu J, Ning XY, et al. Synthetic biological system construction and green intelligent biological manufacturing. CIESC J, 2020, 71(9): 3979-3994 (in Chinese). |
| [84] | 丁明珠, 李炳志, 王颖, 等. 合成生物学重要研究方向进展. 合成生物学, 2020, 1(1): 7-28. Ding MZ, Li BZ, Wang Y, et al. Significant research progress in synthetic biology. Synth Biol J, 2020, 1(1): 7-28 (in Chinese). |
| [85] | Tung QN, Van Loi V, Busche T, et al. Stable integration of the Mrx1-roGFP2 biosensor to monitor dynamic changes of the mycothiol redox potential in Corynebacterium glutamicum. Redox Biol, 2019, 20: 514-525. DOI:10.1016/j.redox.2018.11.012 |
| [86] | Binder D, Frohwitter J, Mahr R, et al. Light-controlled cell factories: employing photocaged isopropyl-β-d-thiogalactopyranoside for light-mediated optimization of lac promoter-based gene expression and (+)-valencene biosynthesis in Corynebacterium glutamicum. Appl Environ Microbiol, 2016, 82(20): 6141-6149. DOI:10.1128/AEM.01457-16 |
| [87] | Kobayashi S, Kawaguchi H, Shirai T, et al. Automatic redirection of carbon flux between glycolysis and pentose phosphate pathway using an oxygen-responsive metabolic switch in Corynebacterium glutamicum. ACS Synth Biol, 2020, 9(4): 814-826. DOI:10.1021/acssynbio.9b00493 |
| [88] | Tan SY, Shi F, Liu HY, et al. Dynamic control of 4-hydroxyisoleucine biosynthesis by modified L-isoleucine biosensor in recombinant Corynebacterium glutamicum. ACS Synth Biol, 2020, 9(9): 2378-2389. DOI:10.1021/acssynbio.0c00127 |
