

安徽大学 生命科学学院,安徽 合肥 230601
收稿日期:2020-06-01;接收日期:2020-07-31
基金项目:国家重点研发计划(No. 2018YFC0311106),国家自然科学基金(No. 31870056) 资助
摘要:从海洋细菌Bacillus sp. D1中克隆、重组表达β-葡萄糖苷酶BglD2,研究其酶学性质,并对其水解虎杖苷制备白藜芦醇的能力进行分析。BglD2的最适催化温度和pH分别为45 ℃和6.5,在30 ℃和pH 6.5条件下的半衰期约为20 h。BglD2能够水解含β (1→3)、β (1→4)、β (1→6) 等键型的多种底物。BglD2具有良好的糖促活特性,100 mmol/L葡萄糖和150 mmol/L木糖分别将酶活力提升2.0倍和2.3倍。BglD2具有较好的乙醇促活及耐受特性,30 ℃时,10%乙醇使酶活力提升1.2倍,25%乙醇存在时其仍保留60%的酶活力。BglD2具有水解虎杖苷制备白藜芦醇的能力,35 ℃条件下反应2 h水解率为86%。具有乙醇耐受及抗产物抑制等特性的β-葡萄糖苷酶BglD2在酶法水解虎杖苷制备白藜芦醇方面有应用潜力。
关键词:β-葡萄糖苷酶葡萄糖耐受乙醇耐受虎杖苷白藜芦醇
Heterologous expression of a novel β-glucosidase BglD2 and its application in polydatin-hydrolyzing
Cheng He, Yan Wu, Chunyu Meng, Yazhong Xiao, Zemin Fang, Wei Fang


School of Life Sciences, Anhui University, Hefei 230601, Anhui, China
Received: June 1, 2020; Accepted: July 31, 2020
Supported by: National Key Research and Development Program (No. 2018YFC0311106), National Natural Science Foundation of China (No. 31870056)
Corresponding author: Wei Fang. Tel: +86-551-63861063; E-mail: fangahu@ahu.edu.cn.
Abstract: A novel β-glucosidase BglD2 with glucose and ethanol tolerant properties was screened and cloned from the deep-sea bacterium Bacillus sp. D1. The application potential of BglD2 toward polydatin-hydrolyzing was also evaluated. BglD2 exhibited the maximal β-glucosidase activity at 45 ℃ and pH 6.5. BglD2 maintained approximately 50% of its origin activity after incubation at 30 ℃ and pH 6.5 for 20 h. BglD2 could hydrolyze a variety of substrates containing β (1→3), β (1→4), and β (1→6) bonds. The activity of β-glucosidase was enhanced to 2.0 fold and 2.3 fold by 100 mmol/L glucose and 150 mmol/L xylose, respectively. BglD2 possessed ethanol-stimulated and -tolerant properties. At 30 ℃, the activity of BglD2 enhanced to 1.2 fold in the presence of 10% ethanol and even remained 60% in 25% ethanol. BglD2 could hydrolyze polydatin to produce resveratrol. At 35 ℃, BglD2 hydrolyzed 86% polydatin after incubation for 2 h. Thus, BglD2 possessed glucose and ethanol tolerant properties and can be used as the potential candidate of catalyst for the production of resveratrol from polydatin.
Keywords: β-glucosidaseglucose toleranceethanol tolerancepolydatinresveratrol
白藜芦醇是一种非黄酮类多酚化合物,具有显著的抗氧化活性,在预防癌症、保护心血管和神经系统、提高免疫力、延长寿命等方面有重要作用[1]。虽然白黎芦醇的药用价值极高,但目前的产量远远不能满足市场需求。白藜芦醇广泛存在于花生、葡萄、虎杖和决明子等天然植物中,但含量均较低。虎杖是白藜芦醇含量最高的来源植物,其中白藜芦醇含量仅为0.2%–0.4%,白黎芦醇与葡萄糖的结合物虎杖苷含量约为1.5%–3.0%[1]。研究表明,高效水解虎杖苷中的糖苷键获得白藜芦醇,是提高白藜芦醇产量的有效途径之一。
酸水解、微生物转化和酶转化等方法能够将虎杖苷转化为白藜芦醇[2-6],其中通过β-葡萄糖苷酶催化转化虎杖苷转获得白藜芦醇具有反应条件温和、操作简单、成本低、污染少等优点。β-葡萄糖苷酶(EC 3.2.1.21),即β-D-葡萄糖苷葡萄糖水解酶,主要水解糖苷或寡糖中的β-1, 4-糖苷键,同时释放葡萄糖以及糖苷配体[7],在现代工业生物技术如食品、医药、饲料加工、能源炼制等领域具有重要应用价值。
β-葡萄糖苷酶能够有效水解虎杖苷中的β-糖苷,生成白藜芦醇,是酶转化制备白藜芦醇的重要用酶。乙醇是虎杖苷提取的常用试剂,虎杖苷提取物作为酶转化底物可能会将一定量的乙醇带入反应体系,而且随着反应的进行,产物葡萄糖会不断累积。因此,具有乙醇耐受及抗产物抑制等活性的β-葡萄糖苷酶将更适合于酶法转化虎杖苷以制备白藜芦醇。获取具有优良催化性能的β-葡萄糖苷酶,将有助于改进现有酶参与的催化水解工艺,从而降低工业生产成本[8]。
海洋是筛选具有盐耐受、有机溶剂耐受、抗碳水化合物抑制等活性的新型β-葡萄糖苷酶的重要来源。前期,从南海海底沉积物中筛选得到一株具有β-葡萄糖苷酶活性的芽孢杆菌属细菌Bacillus sp. D1,与分离自海洋的褐藻降解菌Bacillus weihaiensis Alg07亲缘关系较近。对B. weihaiensis Alg07基因组分析,可知其基因组中含有2个可能的β-葡萄糖苷酶编码基因。依照B. weihaiensis Alg07基因组中β-葡萄糖苷酶的编码基因设计引物,以Bacillus sp. D1基因组为模板,成功克隆并异源表达了β-葡萄糖苷酶BglD1 (GenBank登录号:QCQ29109),该酶显示出独特转糖苷特性[9]。进一步分析B. weihaiensis Alg07基因组获知,基因组中存在另一个β-葡萄糖苷酶基因(GenBank登录号:WP_072580823)。基于此分析,本文依照β-葡萄糖苷酶的编码基因(WP_072580823) 设计引物,以Bacillus sp. D1基因组为模板,克隆并异源表达了新型β-葡萄糖苷酶BglD2,BglD2具有较好的乙醇耐受性及葡萄糖耐受性,能够有效水解虎杖苷制备白藜芦醇,在酶法制备白藜芦醇方面具有潜在的应用价值。
1 材料与方法1.1 材料Bacillus sp. D1菌株为前期从南海海底沉积物样品中筛选获得并保存于实验室。大肠杆菌Escherichia coli BL21(DE3) 感受态细胞,pEASY- T3载体购自北京全式金生物技术有限公司。虎杖苷、白藜芦醇、纤维二糖、葡萄糖、昆布二糖、龙胆二糖、龙胆三糖和纤维三糖等购自Sigma- Aldrich (St. Louis,MO,USA)。p-nitrophenyl- β-D-glucopyranoside (pNPGlc) 购自阿拉丁生化科技股份有限公司。其他试剂为国产或进口分析纯。
1.2 β-葡萄糖苷酶BglD2的克隆及序列分析基于B. weihaiensis Alg07基因组序列分析,以β-葡萄糖苷酶(WP_072580823) 编码基因为参照,设计上游引物5′-GATATACATATGACTAG ATTTTCAAAAGA-3′ (下划线为NdeⅠ酶切位点),下游引物5′-GTGGTGCTCGAGAATGAGA ATCTTTTCCAA-3′ (下划线为XhoⅠ酶切位点),以Bacillus sp. D1基因组为模板扩增目的基因,PCR产物连接pEASY-T3载体后转化至E. coli DH5α感受态细胞,筛选阳性克隆并测序验证序列正确性。以NdeⅠ和XhoⅠ酶切阳性克隆,并连接至pET-22b(+) 表达载体,构建重组质粒pET-22b(+)-bglD2,转化至E. coli BL21(DE3) 感受态细胞。
在NCBI数据库(https://www.ncbi.nlm.nih.gov/) 采用BLAST检索与BglD2序列一致性较高的β-葡萄糖苷酶序列,使用ClustalX以及GENEDOC软件,对BglD2与部分已知β-葡萄糖苷酶序列进行全序列比对分析。
1.3 β-葡萄糖苷酶BglD2的重组表达及纯化将含有pET-22b(+)-bglD2表达载体的E. coli BL21(DE3) 培养至OD600为0.6,加入IPTG至终浓度为0.2 mmol/L诱导,16 ℃培养16 h。4 ℃、6 000×g离心5 min,收集细胞。用150 mL缓冲液(20 mmol/L Tris-HCl,500 mmol/L NaCl,5 mmol/L咪唑,pH 7.8) 重悬菌体,超声破碎。4 ℃、12 000×g离心30 min收集上清,制备粗酶液。Ni2+-NTA亲和柱纯化蛋白,过程参照Novagen使用说明书,纯化的蛋白利用SDS-PAGE检测纯度及分子量,BCA法测定蛋白浓度。
1.4 β-葡萄糖苷酶BglD2的酶活测定以Citrate-Na2HPO4缓冲溶液(50 mmol/L,pH 6.5)配制0.1 mol/L pNPGlc。500 μL反应体系包含25 μL适当稀释的酶液,终浓度为5 mmol/L的pNPGlc和Citrate-Na2HPO4缓冲溶液(50 mmol/L,pH 6.5)。反应体系于45 ℃反应10 min后,加入500 μL Na2CO3 (1 mol/L) 混合均匀终止反应,测定OD405处吸光值。酶活力单位定义为1 min产生1 μmol/L pNP所用的酶量为1 U。
1.5 温度和pH对β-葡萄糖苷酶BglD2酶活力的影响将酶分别置于25–55 ℃ (间隔5 ℃) 条件下,测定不同反应温度对酶活力的影响。温度稳定性测定使用Citrate-Na2HPO4缓冲液(50 mmol/L,pH 6.5) 适当稀释酶液,在30 ℃条件下,间隔一定的时间取样测酶活力。以初始酶活力作为100%,计算相对酶活力。
以5 mmol/L pNPGlc为底物,分别在50 mmol/L Citrate-Na2HPO4缓冲液(pH 4.5–7.0)和HAC-NaAc缓冲液(pH 4.5–5.5) 中测定酶活力,确定反应最适pH。将适量酶液置于不同pH的Citrate- Na2HPO4缓冲液(50 mmol/L,pH 6.0–7.0)中,于45 ℃条件下孵育,测定不同pH条件对酶稳定性的影响。间隔相同的时间取样测酶活力,以初始酶活力作为100%,计算相对酶活力。
1.6 金属离子及化学试剂对酶活力的影响用Citrate-Na2HPO4缓冲液(50 mmol/L,pH 6.5) 分别配制0.1 mol/L K+、Mn2+、Mg2+、Ca2+、Na+、Cu2+、Zn2+、DTT、EDTA、SDS、Urea等溶液。以5 mmol/L pNPGlc为底物,向反应体系中分别加入上述溶液,至终浓度为1 mmol/L和5 mmol/L,在最适条件下测酶活力,以不加上述溶液的酶活作为100%,计算相对酶活力。
1.7 BglD2水解二糖及虎杖苷的酶活力测定以Citrate-Na2HPO4缓冲液(50 mmol/L,pH 6.5) 分别配制0.1 mol/L龙胆二糖、乳糖、纤维二糖和昆布二糖。二糖作为底物时采用500 μL反应体系,包括25 μL适当稀释的酶液和475 μL含有相应浓度底物的Citrate-Na2HPO4缓冲液(50 mmol/L,pH 6.5),45 ℃反应10 min。依照产品说明书,采用葡萄糖氧化酶-过氧化物酶法测定反应体系中葡萄糖含量(Shanghai Rongsheng Biotech Co., Ltd., China)。
以Citrate-Na2HPO4缓冲溶液(50 mmol/L,pH 6.5) 配制80%乙醇,用80%乙醇配制0.1 mol/L虎杖苷母液。500 μL反应体系包含25 μL适当稀释的酶液,终浓度为5 mmol/L的虎杖苷和Citrate-Na2HPO4缓冲溶液(50 mmol/L,pH 6.5)。反应体系于45 ℃反应10 min后,加入500 μL无水乙醇混合均匀终止反应,12 000×g离心5 min,取上清过0.22 μm有机系滤器,高效液相色谱法(High-performance liquid chromatography,HPLC)检测产物生成。酶活力单位定义为1 s产生1 nmol/L白藜芦醇所用的酶量为1 U。
1.8 葡萄糖和木糖对酶活力的影响以Citrate-Na2HPO4缓冲液(50 mmol/L,pH 6.5) 配制葡萄糖和木糖溶液。反应体系中分别加入终浓度为0–2 mol/L的葡萄糖或木糖,在最适条件下测定酶活力。以不加糖的酶活力为100%,计算相对酶活。
1.9 BglD2转糖苷活性的测定建立2.5 mL的转糖苷反应体系,包含酶液500 μL (10 U/mL,以pNPGlc为底物测定),终浓度为150 g/L的纤维二糖,35 ℃条件下反应2 h,采用HPLC法检测转糖苷产物。
1.10 乙醇对酶活力及稳定性的影响以pNPGlc为底物,向反应体系中分别加入终浓度为5%–30%的乙醇,分别在30–50 ℃条件下,测定不同浓度乙醇对酶活力的影响。以不加乙醇的酶活力为100%,计算相对酶活力。
以pNPGlc为底物,向反应体系中加入10%乙醇,在30 ℃条件下测定乙醇对酶稳定性的影响。以初始酶活力为100%,计算相对酶活力。
1.11 酶动力学分析反应体系中加入终浓度为0–3 mmol/L的pNPGlc为底物,在最适反应条件下,测定BglD2反应动力学参数。反应体系中分别加入终浓度为10%的乙醇、100 mmol/L的葡萄糖和150 mmol/L的木糖,分别测定乙醇、葡萄糖、木糖对BglD2动力学参数的影响。使用Origin 8.5软件,通过非线性拟合Michaelis-Menten方程,推算酶催化的Km、kcat及催化效率(kcat/Km)。
1.12 BglD2水解虎杖苷的动力学参数测定体系中加入终浓度为0.02–5.00 mmol/L的虎杖苷作为底物,建立水解反应体系。反应结束以500 μL无水乙醇终止反应,12 000×g离心5 min,取上清过0.22 μm有机系滤器,HPLC检测产物生成。根据标准曲线计算白藜芦醇量,采用Origin 8.5软件非线性拟合Michaelis-Menten方程,计算酶水解虎杖苷动力学参数。
1.13 BglD2水解虎杖苷制备白藜芦醇条件优化以Citrate-Na2HPO4缓冲液(50 mmol/L,pH 6.5) 配制80%乙醇,以配制200 mmol/L虎杖苷母液。建立8 mL酶水解虎杖苷的反应体系,虎杖苷终浓度为2 mg/mL (乙醇浓度为15%),酶量为5 U。将反应体系放入30–45 ℃水浴中反应3 h,以500 μL无水乙醇终止反应,12 000×g离心5 min,上清过0.22 μm有机系滤器,HPLC检测,确定最适反应温度。
最适反应温度下,向8 mL反应体系中加入5–20 U的酶,反应3 h,以500 μL无水乙醇终止反应,12 000×g离心5 min,上清过0.22 μm有机系滤器过滤,HPLC检测,测定反应最适酶量。
最适反应温度和酶量下,在8mL反应体系中分别加入终浓度为2、3、4 mg/mL的虎杖苷(乙醇浓度分别为15%、20%、25%),酶量为15 U,反应3 h,以500 μL无水乙醇终止反应,12 000×g离心5 min,取上清过0.22 μm有机系滤器过滤,HPLC检测,测定反应最适底物浓度。
建立优化的水解反应体系,分别反应1、2、3 h,以500 μL无水乙醇终止反应,12 000×g离心5 min,取上清过0.22 μm有机系滤器过滤,HPLC检测,优化反应时间。
虎杖苷水解率计算公式为:虎杖苷水解率(%)=(水解前虎杖苷质量-水解后虎杖质量)/水解前虎杖苷质量×100。
1.14 高效液相色谱(HPLC) 检测虎杖苷水解产物检测使用Agilent Technologies 1260 Infinity设备,配备蒸发光检测器及TSKgel Amide-80 (4.6 mm×25 cm,5 μm) 分析柱。洗脱流动相A为甲醇,流动相B为水,采用梯度洗脱,30 min内流动相由A︰B为20︰80逐渐过渡至90︰10,维持洗脱5 min;接着在5 min内降至20︰80。流速0.5 mL/min,柱温30 ℃,于303 nm检测产物的生成。
2 结果与分析2.1 β-葡萄糖苷酶BglD2的重组表达及序列分析由Bacillus sp. D1菌株克隆获得bglD2,全长1 347 bp,基因序列已经递交GenBank数据库(登录号:MT815914)。该基因编码由449个氨基酸组成的蛋白,SDS-PAGE结果显示其分子量约为50 kDa (图 1),与基于氨基酸序列计算的理论分子量49.4 kDa相近。以pNPGlc为底物,纯化后蛋白比酶活为(120.90±0.03) U/mg。
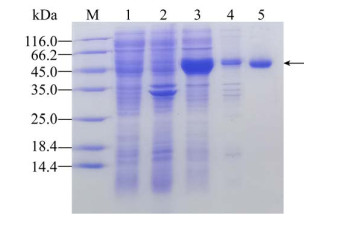 |
| 图 1 β-葡萄糖苷酶BglD2的SDS-PAGE电泳分析 Fig. 1 SDS-PAGE analysis of β-glucosidase BglD2. M: protein molecular weight marker (Thermo Fisher Scientific, Inc.); lane 1: the sonication supernatant of E. coli harboring plasmid pET22b(+)-bglD2 without induction; lane 2: the sonication precipitate of E. coli harboring plasmid pET22b(+)-bglD2 without induction; lane 3: the sonication supernatant of E. coli harboring plasmid pET22b(+)-bglD2 induced by IPTG; lane 4: the sonication precipitate of E. coli harboring plasmid pET22b(+)-bglD2 induced by IPTG; lane 5: the protein after purification by Ni2+-NTA affinity chromatography. |
| 图选项 |
序列分析表明,与BglD2氨基酸序列一致性超过70%的蛋白序列,多为基因组测序或直接递交。BglD2与部分来自海洋的β-葡萄糖苷酶序列一致性较高,如Bacillus weihaiensis Alg07 (一致性99%,相似性99%)、Bacillus mesophilus (一致性82%,相似性90%)、海洋芽孢杆菌Bacillus oceanisediminis (一致性81%,相似性90%),这些序列均来自全基因组测序。与部分已性质表征的GH1家族β-葡萄糖苷酶相比,BglD2与来自表层海水微生物元基因组文库的β-葡萄糖苷酶Bgl1A的序列一致性为44% (相似性60%),来自同一文库的Bgl1B的序列一致性为40% (相似性60%)[10-11]。BglD2与前期从Bacillus sp. D1菌株克隆获得的β-葡萄糖苷酶BglD1的一致性为47%,相似性为63%。序列比对发现,BglD2与部分已性质表征的GH1家族β-葡萄糖苷酶都具有该家族相对保守的序列NEP和ENG[10],其中Glu163和Glu351为BglD2推测的催化残基(图 2)。
 |
| 图 2 BglD2与部分已知β-葡萄糖苷酶序列比对 Fig. 2 Alignments of the amino acid sequences of BglD2 and other known β-glucosidases. The conserved motifs of NEP and ENG were boxed. ADD 96762: β-glucosidase Bgl1A from metagenomic library; ACY 09072: β-glucosidase Bgl1B from metagenomic library; WP 072580823: β-glucosidase from Bacillus weihaiensis Alg07; QCQ29109: β-glucosidase BglD1 from Bacillus sp. D1. |
| 图选项 |
2.2 温度和pH对β-葡萄糖苷酶BglD2酶活力的影响β-葡萄糖苷酶BglD2最适反应温度为45 ℃,在25–50 ℃范围内保持超过50%的酶活力(图 3A)。BglD2的最适温度与一些来自海洋的微生物酶类似,均处于45–55 ℃温度区间,如来自气单胞菌属Aeromonas sp. 、链霉菌属Streptomycete sp. 、地中海马特尔氏菌Martelella mediterranea的酶[12-14],当反应温度提高时,因酶的稳定性下降而活性降低。BglD2最适反应pH为6.5,在pH 5.5–7.0范围内保持超过80%的酶活力(图 3B)。与海洋来源酶类似,BglD2高温不稳定,30 ℃半衰期可延长至20 h (图 3C)。BglD2在pH 6.0–7.0范围内较稳定,pH 7.0时的稳定性最好(图 3D)。
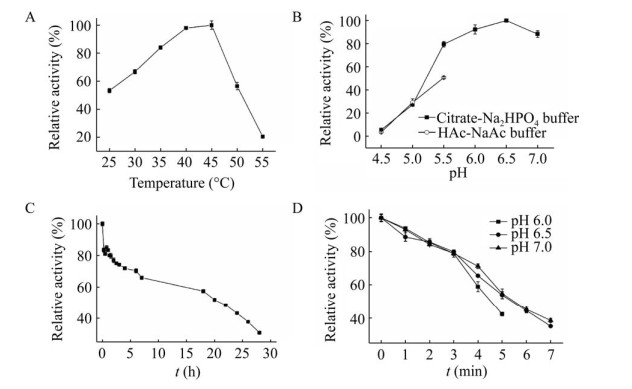 |
| 图 3 温度和pH对BglD2酶活力及稳定性的影响 Fig. 3 Effects of temperature and pH on the activity (A and B) and stability (C and D) of BglD2. The optimum temperature and pH were determined with pNPGlc as the substrate. For the effect of temperature on enzyme stability, the purified enzyme was kept at 30 ℃ in 50 mmol/L Citrate-Na2HPO4 buffer (pH 6.5), and the residual activity was measured using pNPGlc as the substrate. For the effect of pH on enzyme stability, the purified enzyme was pre–incubated in 50 mmol/L Citrate-Na2HPO4 buffer (pH 6.0 to pH 7.0) at 45 ℃, and the remaining activities were measured with pNPGlc as the substrate. All the results were the average of triplicate experiments. |
| 图选项 |
2.3 金属离子及化学试剂对酶活力的影响5 mmol/L的Cu2+、SDS对酶活力均有抑制作用,5 mmol/L的Mg2+、Ca2+、Na+、DTT对酶活力均有促进作用,其中DTT可使酶活力提升至1.27倍(表 1)。EDTA的加入对酶活力无影响,由此推测BglD2不是金属离子依赖性的蛋白,与多数已报道的β-葡萄糖苷酶结果一致[15-17]。
表 1 金属离子及化学试剂对BglD2酶活力的影响Table 1 Effects of metal ions and chemical reagents on BglD2 activity
| Metal ions | Relative activity (%) | |
| 1 mmol/L | 5 mmol/L | |
| K+ | 100.67±1.81 | 91.75±3.59 |
| Mn2+ | 99.54±2.56 | 92.09±9.29 |
| Ca2+ | 106.66±1.02 | 113.20±5.84 |
| Mg2+ | 95.79±3.63 | 111.12±2.89 |
| Na+ | 100.58±1.19 | 112.37±1.13 |
| Cu2+ | 96.67±4.13 | 79.26±2.90 |
| Zn2+ | 95.25±3.55 | 89.96±4.82 |
| DTT | 102.29±1.51 | 127.70±3.15 |
| EDTA | 96.92±2.60 | 100.46±1.68 |
| SDS | 40.02±3.16 | 21.99±1.02 |
| Urea | 105.41±3.43 | 101.33±2.58 |
表选项
2.4 BglD2水解虎杖苷和二糖底物的比酶活测定以虎杖苷为底物,BglD2催化比酶活为(284.05±2.47) U/mg。BglD2可以水解β (1→4)、β (1→3)、β (1→6)等多种键型。以天然二糖为底物,BglD2对纤维二糖和昆布二糖的水解活力相对较高。与来自同一菌株的β-葡萄糖苷酶BglD1不同[9],BglD2对乳糖的水解能力较弱(表 2)。
表 2 BglD2水解二糖底物的比酶活Table 2 Specific activity of BglD2 toward disaccharide substrates
| Substrates | Linkages | Enzyme activity (U/mg) | Relative activity (%) |
| Cellobiose | β (1→4) | 4.02±0.08 | 100 |
| Laminaribiose | β (1→3) | 3.63±0.18 | 90 |
| Gentiobiose | β (1→6) | 1.20±0.02 | 30 |
| Lactose | β (1→4) | 0.49±0.02 | 12 |
表选项
2.5 葡萄糖和木糖对酶活力的影响葡萄糖和木糖对BglD2的酶活力有一定的促进作用,100 mmol/L的葡萄糖对酶活力促进作用最大,达到原酶活力的2.0倍;150 mmol/L的木糖对酶活力的促进作用最大,达到原酶活力的2.3倍。随着糖浓度的增加,酶活力受到抑制。当木糖和葡萄糖浓度提升至1.2 mol/L和1.0 mol/L,BglD2酶活力降低为原酶活力的一半(图 4)。
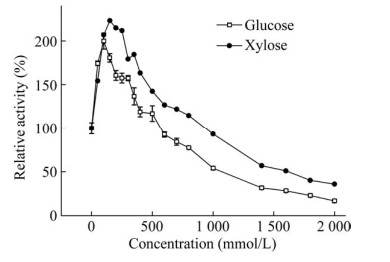 |
| 图 4 葡萄糖和木糖对BglD2酶活力的影响 Fig. 4 Effects of glucose and xylose on the activity of BglD2 with pNPGlc as the substrate. The activity of the enzyme was evaluated in the presence of varying concentrations of monosaccharides. The relative activity was defined as the activity in the presence of effectors relative to that in the absence of the effectors. All results were the average of triplicate experiments. |
| 图选项 |
已有研究发现,糖对酶活力的促进与酶的转糖苷活性有关。以纤维二糖为底物,经BglD2转糖苷后生成纤维三糖,表明BglD2具有一定的转糖苷能力(图 5)。
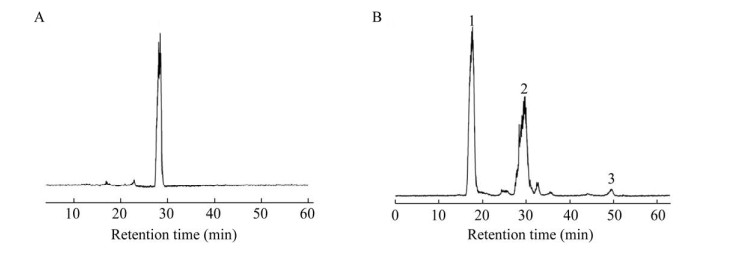 |
| 图 5 以纤维二糖为底物的转糖苷产物检测 Fig. 5 HPLC analysis of transglycosylation activity with cellobiose as the substrate. (A) Cellobiose in the reaction mixture before transglycosylation. Peak 1, cellobiose. (B) Transglycosylation products of BglD2. Peaks: 1 glucose, 2 cellobiose, 3 cellotriose. |
| 图选项 |
2.6 乙醇对酶活力及稳定性的影响30 ℃条件下,10%乙醇对BglD2的酶活力具有促进作用,可使酶活力提升约1.2倍,25%的乙醇中可保留约60%酶活力。当温度升高至45 ℃以上,乙醇对酶活力的促进作用减弱(图 6A)。乙醇对酶的稳定性影响较大,30 ℃条件下,BglD2在10%乙醇中的半衰期仅为5 h,而不加乙醇时的半衰期大约为20 h (图 6B)。
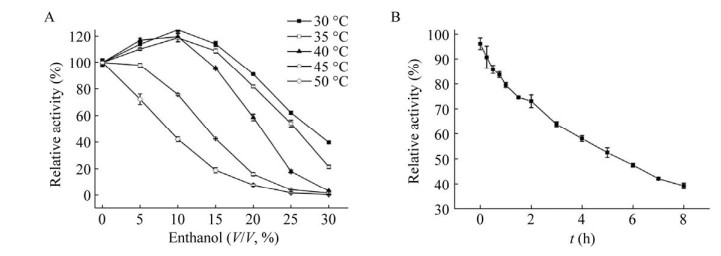 |
| 图 6 乙醇对BglD2酶活力(A) 及稳定性(B) 的影响 Fig. 6 Effects of ethanol on the enzyme activity and stability. (A) Effects of ethanol on the enzyme activity. Reaction mixtures contained 5 mmol/L pNPGlc in Citrate-Na2HPO4 buffer (50 mmol/L, pH 6.5) with varying concentrations of ethanol. After incubation at 30–50 ℃ for 10 min, the activity was measured under standard assay conditions. (B) Effect of ethanol on the stability of BglD2. The stability was assessed by incubating appropriate volumes of the purified enzyme with 10% (V/V) ethanol at 30 ℃. Aliquots were taken at regular interval, and the residual activity was monitored using pNPGlc as the substrate. All figures were the average of results from triplicate experiments. |
| 图选项 |
2.7 酶动力学参数在最适反应条件下,以pNPGlc为底物时,Km和kcat分别为0.23 mmol/L和103.04 s–1 (表 3)。一定浓度的木糖和葡萄糖对BglD2的酶活力有一定的促进,动力学分析亦表明,在100 mmol/L葡萄糖或150 mmol/L木糖存在时,酶催化的kcat分别增加了136%和167% (表 3)。在10%乙醇存在条件下,酶催化kcat增加了11%。
表 3 BglD2动力学参数Table 3 Kinetic parameters of BglD2
| Km (mmol/L) | kcat (s–1) | kcat/Km (L/(mmol·s)) | |
| pNPGlc | 0.23±0.02 | 103.04±0.02 | 444.17±0.01 |
| pNPGlc (10% ethanol) | 1.14±0.02 | 114.37±0.03 | 100.32±0.02 |
| pNPGlc (100 mmol/L glucose) | 0.71±0.04 | 244.52±0.02 | 316.27±0.01 |
| pNPGlc (150 mmol/L xylose) | 0.69±0.04 | 276.27±0.01 | 397.51±0.02 |
| Polydatin | 1.03±0.01 | 12.95±0.01 | 12.57±0.01 |
表选项
以虎杖苷为底物,BglD2水解虎杖苷的Km为1.03 mmol/L (表 3),稍高于来自Aspergillus niger SK34.002的β-葡萄糖苷酶水解虎杖苷的Km (0.74 mmol/L)[18]。
2.8 虎杖苷水解条件优化以白藜芦醇生成量为衡量指标,BglD2在30–35 ℃范围内,白藜芦醇产量较高,超过35 ℃,产量下降,因此后续优化在35 ℃条件下进行(图 7A)。酶量优化结果显示,加入1.8 U/mL BglD2白藜芦醇产量最高(图 7B)。底物浓度优化结果表明,加入3 mg/mL虎杖苷,水解后白藜芦醇产量最高(图 7C)。时间优化结果表明,水解反应2 h后白藜芦醇产量最高(图 7D)。优化后BglD2水解虎杖苷反应条件为:底物浓度3 mg/mL,1.8 U/mL酶量,在35 ℃条件下反应2 h。最优条件下,BglD2对虎杖苷的水解率达到86%,白藜芦醇产量达到0.75 mg/mL (图 7)。
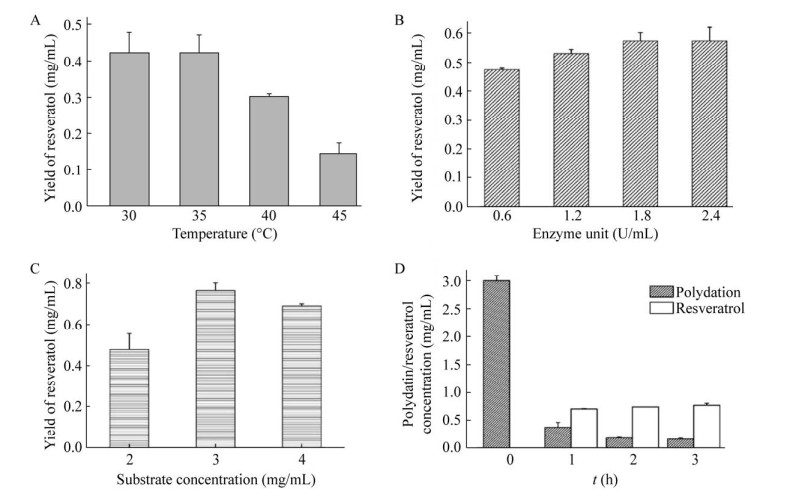 |
| 图 7 BglD2水解虎杖苷制备白藜芦醇条件优化 Fig. 7 Hydrolysis of polydatin for the production of resveratrol by BglD2. (A) Effect of temperature on the production of resveratrol. (B) Effect of enzyme dosage on the production of resveratrol. (C) Effect of substrate concentration on the yield of resveratrol. (D) Time course of the reaction mixture catalyzed by BglD2. All results were the average of triplicate experiments. |
| 图选项 |
3 讨论来自降解藻类微生物的糖苷水解酶可能具有与陆地来源酶截然不同的特点,因而被认为是挖掘新颖酶资源的宝库[19]。作为与褐藻降解菌B. weihaiensis Alg07的16S rRNA序列一致性较高的微生物,Bacillus sp. D1基因组中可能亦蕴含了新型糖苷水解酶的编码基因。前期,已从Bacillus sp. D1基因组成功克隆并表达了新型β-葡萄糖苷酶BglD1,该酶具有较强的转糖苷活性,并适用于低聚半乳糖的合成。本研究从Bacillus sp. D1基因组中,成功克隆了另一个新型β-葡萄糖苷酶BglD2,与BglD1的序列一致性仅为47%,且两者的酶学性质差异较大,说明BglD1和BglD2在来源菌Bacillus sp. D1中可能行使了不同的生物学功能。
海洋来源、具有中温催化特性的β-葡萄糖苷酶,其最适催化温度多在45–55 ℃范围[13-14, 20],如来自海洋表层海水微生物元基因组文库的Bgl1A[10]和Bgl1B[11],来自Bacillus sp. D1的BglD1[9]和BglD2等。虽然此类酶拥有高于海洋环境的最适催化温度,但高温下多不稳定[9-10]。BglD2在pH 6.0–7.0环境中具有比较高的酶活力且比较好的稳定性,符合细菌β-葡萄糖苷酶的催化特性[11],这一特性在海洋来源酶Bgl1A[10]、Bgl1B[11]和BglD1[9]等酶中亦有体现。一般而言,低浓度的Cu2+是β-葡萄糖苷酶的有效抑制剂,而其他金属离子对酶活力的影响各异。对于BglD2,Mg2+、Ca2+、Na+等金属离子可提升酶活力至1.1倍以上,而对来自同一菌株的BglD1的影响不同,仅有Mg2+可提升BglD1的酶活力,Ca2+和Na+对酶活影响较小[9]。
已知β-葡萄糖苷酶普遍存在受产物葡萄糖反馈抑制,如现有的绝大多数β-葡萄糖苷酶都对其水解反应产物葡萄糖异常敏感,极低浓度的葡萄糖就可以反馈抑制β-葡萄糖苷酶的活性,其抑制常数(Ki) 一般为0.5–100 mmol/L。但亦有多种属来源的具有葡萄糖耐受性的β-葡萄糖苷酶被报道,一些酶的葡萄糖耐受Ki可达摩尔级,并且往往伴随着葡萄糖对酶的激活作用[10, 21-23]。此外,木糖、蔗糖、半乳糖等对β-葡萄糖苷酶的酶活力也显示出促进作用[24-26]。来自Bacillus sp. D1的BglD2和BglD1,以及来自海洋表层海水微生物元基因组文库的Bgl1A[10]均具有糖促活特性,葡萄糖、木糖能够有效地促进酶活力的提升[9]。转糖苷作用在糖促进β-葡萄糖苷酶酶活提升方面起非常重要的作用[27-28]。以纤维二糖为底物,BglD1显示出了良好的转糖苷活性[9],BglD2、Bgl1A[10]亦显示较弱的转糖苷活性,由此推测,转糖苷作用在β-葡萄糖苷酶受糖促活方面可能也起到了重要作用。
少数β-葡萄糖苷酶显示出乙醇耐受的特性,如来自土壤元基因组文库的β-葡萄糖苷酶Bg10,在500 mmol/L乙醇条件下可保持大于70%的酶活力;来自解纤维芽孢杆菌Bacillus cellulosilyticus的β-葡萄糖苷酶的乙醇耐受Ki为15% (V/V)[29];来自海洋元基因组文库的β-葡萄糖苷酶Bgl1A,经过基因工程改造,乙醇耐受性得以提升[30]。与已报道的乙醇耐受特性不同,BglD2显示出良好的乙醇促活特性,10%的乙醇可使酶活力提升至1.2倍,在此条件下酶催化kcat值有所增加。短侧链醇对β-葡萄糖苷酶活力的促进在嗜热毁丝霉Myceliophthora thermophila[31]、嗜热子囊菌Thermoascus aurantiacus[32]、米曲霉Aspergillus oryzae[33]以及尖孢镰刀菌Fusarium oxysporum[34]等来源的酶中亦有发现,对这一现象的可能解释为乙醇引起的溶液极性的变化可以一定程度上稳定酶的构象[35]。
以虎杖苷为底物,β-葡萄糖苷酶能够有效水解糖苷键,释放苷元。用于虎杖苷酶法转化生成白藜芦醇的β-葡萄糖苷酶多来源于真菌,如布鲁塞尔德克酵母Dekkera bruxellensis[36-37]、酿酒酵母Saccharomyces cerevisiae[38]、Aspergillus oryzae[39]和黑曲霉Aspergillus niger[38],以及细菌,如乳酸杆菌属Lactobacillus sp. [40]和Bacillus sp. [41]等来源的酶。表 4列举了已报道用于水解虎杖苷的β-葡萄糖苷酶,除Bgl2238外,均由来源菌发酵制备,酶的基因或蛋白序列未见报道。β-葡萄糖苷酶Bgl2238蛋白基因(GenBank登录号:KU320675) 克隆自土壤元基因组文库[42],序列分析表明其为GH3家族β-葡萄糖苷酶,与BglD2分属不同糖苷水解酶家族。
表 4 BglD2与已报道β-葡萄糖苷酶水解虎杖苷的比酶活、Km及水解条件比较Table 4 Comparison of the specific activity, Km and hydrolysis conditions toward polydatin using BglD2 and some known β-glucosidases
| Enzyme origin | Specific activity (U/mg) | Km (mmol/L) | Temperature (℃) | pH | Time | Hydrolysis rates (%) | References |
| Aspergillus oryzae sp. 100 | 55.00 | 0.74 | 60 | 5.0 | 2 h | About 100% | [39] |
| Aspergillus oryzae (CICC 2436) | Nm | Nm | 50 | 4.8 | 8 h | About 100% | [5] |
| Aspergillus niger SK34.002 | 75.90 | 0.74 | 60 | Nm | 4 h | About 100% | [18] |
| Dekkera bruxellensis (BCRC920084) | 115.00 | Nm | 50 | 5.0 | About 25 h | About 80% | [36] |
| Almonds (Sigma) | 54.15 | Nm | 50 | 6.0 | About 12 h | About 100% | [36] |
| Lactobacillus kimchi JB301 | 1.49a | 0.20 | 30–40 | 5.0 | 40 h | Over 99% | [40] |
| Bgl2238 from metagenomic library | Nm | Nm | 44 | Nm | 12 h | 95% | [42] |
| Bacillus sp. D1 | 284.05 | 1.03 | 35 | 6.5 | 2 h | 86% | This study |
| a: one unit of enzyme activity was defined as the amount of enzyme capable of hydrolyzing 1 μmol of glucose from glucosides per minute. Nm: not mentioned. | |||||||
表选项
以虎杖苷为底物,BglD2显示出较高的比酶活,是Dekkera bruxellensis来源酶的2.5倍,但催化Km稍高于Aspergillus sp. 来源酶(表 4)。部分真菌β-葡萄糖苷酶,如Aspergillus sp. 以及Dekkera bruxellensis来源酶,需要在高于50 ℃条件下水解虎杖苷生成白藜芦醇(表 4)。虽然多种属来源的β-葡萄糖苷酶在水解虎杖苷方面显示出较高的水解率,但有些酶需要较长的反应时间,如Aspergillus oryzae (CICC 2436) 来源酶需要8 h左右可达到100%水解率,Dekkera bruxellensis来源酶则需要25 h左右,可达80%以上水解率(表 4)。相比而言,来源于海洋细菌Bacillus sp. D1的BglD2,可在35 ℃条件下水解虎杖苷,反应2 h后水解率达到86%,具有低温、高效催化虎杖苷水解的优势。另一方面,水解体系中,虎杖苷提取物作为酶转化底物将一定量的乙醇带入反应体系,而且随着反应的进行,产物葡萄糖会不断累积。具有乙醇耐受及抗产物抑制等特性的β-葡萄糖苷酶BglD2将更适合于酶法转化虎杖苷以制备白藜芦醇。
4 结论海洋是筛选具有盐耐受、有机溶剂耐受、抗碳水化合物抑制等活性的新型β-葡萄糖苷酶的重要来源。本研究从海洋细菌Bacillus sp. D1中成功克隆并异源表达了β-葡萄糖苷酶BglD2,该酶具有较好的乙醇及葡萄糖促活特性,能够在较短时间、较低温度中快速水解虎杖苷生成白藜芦醇,具有酶法生产白藜芦醇的潜力。
参考文献
| [1] | 周林芳. 酶法提取虎杖中白藜芦醇及白藜芦醇酯的合成研究[D]. 无锡: 江南大学, 2019. Zhou LF. Study on enzymatic extraction of resveratrol from Polygonum cuspidatum and synthesis of resveratrol esters[D]. Wuxi: Jiangnan University, 2019 (in Chinese). |
| [2] | Jin S, Luo M, Wang W, et al. Biotransformation of polydatin to resveratrol in Polygonum cuspidatum roots by highly immobilized edible Aspergillus niger and yeast. Bioresour Technol, 2013, 136: 766-770. DOI:10.1016/j.biortech.2013.03.027 |
| [3] | Tian TL, Sun QL, Shen J, et al. Microbial transformation of polydatin and emodin-8-β-d-glucoside of Polygonum cuspidatum Sieb. et Zucc into resveratrol and emodin respectively by Rhizopus microsporus. World J Microbiol Biotechnol, 2008, 24(6): 861-866. DOI:10.1007/s11274-007-9551-z |
| [4] | La Torre GL, Lagana G, Bellocco E, et al. Improvement on enzymatic hydrolysis of resveratrol glucosides in wine. Food Chem, 2004, 85(2): 259-266. DOI:10.1016/j.foodchem.2003.06.019 |
| [5] | Wang H, Liu L, Guo YX, et al. Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae. Appl Microbiol Biotechnol, 2007, 75(4): 763-768. DOI:10.1007/s00253-007-0874-3 |
| [6] | Yang C, Yan AX, Yang XY, et al. An optimum fermentation model established by genetic algorithm for biotransformation from crude polydatin to resveratrol. Appl Biochem Biotechnol, 2012, 166(2): 446-457. DOI:10.1007/s12010-011-9440-7 |
| [7] | Li D, Li XL, Dang W, et al. Characterization and application of an acidophilic and thermostable β-glucosidase from Thermofilum pendens. J Biosci Bioeng, 2013, 115(5): 490-496. DOI:10.1016/j.jbiosc.2012.11.009 |
| [8] | Pei JJ, Pang Q, Zhao LG, et al. Thermoanaerobacterium thermosaccharolyticum β-glucosidase: a glucose-tolerant enzyme with high specific activity for cellobiose. Biotechnol Biofuels, 2012, 5: 31. DOI:10.1186/1754-6834-5-31 |
| [9] | Deng PJ, Meng CY, Wu Y, et al. An unusual GH1 β-glucosidase from marine sediment with β-galactosidase and transglycosidation activities for superior galacto-oligosaccharide synthesis. Appl Microbiol Biotechnol, 2020, 104(11): 4927-4943. DOI:10.1007/s00253-020-10578-z |
| [10] | Fang ZM, Fang W, Liu JJ, et al. Cloning and characterization of a β-glucosidase from marine microbial metagenome with excellent glucose tolerance. J Microbiol Biotechnol, 2010, 20(9): 1351-1358. DOI:10.4014/jmb.1003.03011 |
| [11] | 房伟, 方泽民, 刘娟娟, 等. 新型海洋微生物β-葡萄糖苷酶基因的克隆、表达及重组酶性质. 生物工程学报, 2009, 25(12): 1914-1920. Fang W, Fang ZM, Liu JJ, et al. Cloning and characterization of a β-glucosidase from marine metagenome. Chin J Biotech, 2009, 25(12): 1914-1920 (in Chinese). DOI:10.3321/j.issn:1000-3061.2009.12.021 |
| [12] | Wang ZL, Wang JP, Jiang MH, et al. Selective production of rubusoside from stevioside by using the sophorose activity of β-glucosidase from Streptomyces sp. GXT6. Appl Microbiol Biotechnol, 2015, 99(22): 9663-9674. DOI:10.1007/s00253-015-6802-z |
| [13] | Huang XL, Zhao Y, Dai YJ, et al. Cloning and biochemical characterization of a glucosidase from a marine bacterium Aeromonas sp. HC11e-3. World J Microbiol Biotechnol, 2012, 28(12): 3337-3344. DOI:10.1007/s11274-012-1145-8 |
| [14] | Mao XX, Hong YZ, Shao ZZ, et al. A novel cold-active and alkali-stable β-glucosidase gene isolated from the marine bacterium Martelella mediterranea. Appl Biochem Biotechnol, 2010, 162(8): 2136-2148. DOI:10.1007/s12010-010-8988-y |
| [15] | Asha BM, Pathma J, Sakthivel N. Isolation and characterization of a novel thermostable β-glucosidase from Bacillus subtilis SU40. Prikl Biokhim Mikrobiol, 2015, 51(1): 24-29. |
| [16] | Chang KH, Jo MN, Kim KT, et al. Purification and characterization of a ginsenoside Rb1-hydrolyzing β-glucosidase from Aspergillus niger KCCM 11239. Int J Mol Sci, 2012, 13(9): 12140-12152. |
| [17] | Mallerman J, Papinutti L, Levin L. Characterization of β-glucosidase produced by the white rot fungus Flammulina velutipes. J Microbiol Biotechnol, 2015, 25(1): 57-65. DOI:10.4014/jmb.1401.01045 |
| [18] | Zhou LF, Li SH, Zhang T, et al. Properties of a novel polydatin-β-d-glucosidase from Aspergillus niger SK34.002 and its application in enzymatic preparation of resveratrol. J Sci Food Agric, 2016, 96(7): 2588-2595. DOI:10.1002/jsfa.7465 |
| [19] | Martin M, Vandermies M, Joyeux C, et al. Discovering novel enzymes by functional screening of plurigenomic libraries from alga-associated Flavobacteriia and Gammaproteobacteria. Microbiol Res, 2016, 186-187: 52-61. DOI:10.1016/j.micres.2016.03.005 |
| [20] | Mai ZM, Yang J, Tian XP, et al. Gene cloning and characterization of a novel salt-tolerant and glucose-enhanced β-glucosidase from a marine Streptomycete. Appl Biochem Biotechnol, 2013, 169(5): 1512-1522. DOI:10.1007/s12010-012-0080-3 |
| [21] | Uchima CA, Tokuda G, Watanabe H, et al. Heterologous expression and characterization of a glucose-stimulated β-glucosidase from the termite Neotermes koshunensis in Aspergillus oryzae. Appl Microbiol Biotechnol, 2011, 89(6): 1761-1771. DOI:10.1007/s00253-010-2963-y |
| [22] | Chan CS, Sin LL, Chan KG, et al. Characterization of a glucose-tolerant β-glucosidase from Anoxybacillus sp. DT3-1. Biotechnol Biofuels, 2016, 9: 174. DOI:10.1186/s13068-016-0587-x |
| [23] | Crespim E, Zanphorlin LM, De Souza FHM, et al. A novel cold-adapted and glucose-tolerant GH1 β-glucosidase from Exiguobacterium antarcticum B7. Int J Biol Macromol, 2016, 82: 375-380. DOI:10.1016/j.ijbiomac.2015.09.018 |
| [24] | Masui DC, Zimbardi ALRL, Souza FHM, et al. Production of a xylose-stimulated β-glucosidase and a cellulase-free thermostable xylanase by the thermophilic fungus Humicola brevis var. thermoidea under solid state fermentation. World J Microbiol Biotechnol, 2012, 28(8): 2689-2701. DOI:10.1007/s11274-012-1079-1 |
| [25] | Souza FHM, Inocentes RF, Ward RJ, et al. Glucose and xylose stimulation of a β-glucosidase from the thermophilic fungus Humicola insolens: A kinetic and biophysical study. J Mol Catal B: Enzy, 2013, 94: 119-128. DOI:10.1016/j.molcatb.2013.05.012 |
| [26] | Xu H, Xiong AS, Zhao W, et al. Characterization of a glucose-, xylose-, sucrose-, and D-galactose- stimulated β-glucosidase from the alkalophilic bacterium Bacillus halodurans C-125. Curr Microbiol, 2011, 62(3): 833-839. DOI:10.1007/s00284-010-9766-3 |
| [27] | de Giuseppe PO, Souza TACB, Souza FHM, et al. Structural basis for glucose tolerance in GH1 β-glucosidases. Acta Crysta D Crystallogr Biol, 2014, 70(6): 1631-1639. DOI:10.1107/S1399004714006920 |
| [28] | Uchiyama T, Miyazaki K, Yaoi K. Characterization of a novel β-glucosidase from a compost microbial metagenome with strong transglycosylation activity. J Biol Chem, 2013, 288(25): 18325-18334. DOI:10.1074/jbc.M113.471342 |
| [29] | Wu J, Geng AL, Xie RR, et al. Characterization of cold adapted and ethanol tolerant β-glucosidase from Bacillus cellulosilyticus and its application for directed hydrolysis of cellobiose to ethanol. Int J Biol Macromol, 2018, 109: 872-879. DOI:10.1016/j.ijbiomac.2017.11.072 |
| [30] | Fang W, Yang Y, Zhang XM, et al. Improve ethanol tolerance of β-glucosidase Bgl1A by semi-rational engineering for the hydrolysis of soybean isoflavone glycosides. J Biotechnol, 2016, 227: 64-71. DOI:10.1016/j.jbiotec.2016.04.022 |
| [31] | Karnaouri A, Topakas E, Paschos T, et al. Cloning, expression and characterization of an ethanol tolerant GH3 β-glucosidase from Myceliophthora thermophila. Peer J, 2013, 1: e46. DOI:10.7717/peerj.46 |
| [32] | Parry NJ, Beever DE, Owen E, et al. Biochemical characterization and mechanism of action of a thermostable β-glucosidase purified from Thermoascus aurantiacus. Biochem J, 2001, 353(1): 117-127. DOI:10.1042/bj3530117 |
| [33] | Riou C, Salmon JM, Vallier MJ, et al. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol, 1998, 64(10): 3607-3614. DOI:10.1128/AEM.64.10.3607-3614.1998 |
| [34] | Christakopoulos P, Goodenough PW, Kekos D, et al. Purification and characterisation of an extracellular β-glucosidase with transglycosylation and exo-glucosidase activities from Fusarium oxysporum. Eur J Biochem, 2005, 224(2): 379-385. |
| [35] | Mateo JJ, Di Stefano R. Description of the β-glucosidase activity of wine yeasts. Food Microbiol, 1997, 14(6): 583-591. DOI:10.1006/fmic.1997.0122 |
| [36] | Kuo HP, Wang R, Huang CY, et al. Characterization of an extracellular β-glucosidase from Dekkera bruxellensis for resveratrol production. J Food Drug Anal, 2018, 26(1): 163-171. DOI:10.1016/j.jfda.2016.12.016 |
| [37] | Kuo HP, Wang R, Lin YS, et al. Pilot scale repeated fed-batch fermentation processes of the wine yeast Dekkera bruxellensis for mass production of resveratrol from Polygonum cuspidatum. Bioresour Technol, 2017, 243: 986-993. DOI:10.1016/j.biortech.2017.07.053 |
| [38] | Todaro A, Palmeri R, Barbagallo RN, et al. Increase of trans-resveratrol in typical sicilian wine using β-glucosidase from various sources. Food Chem, 2008, 107(4): 1570-1575. DOI:10.1016/j.foodchem.2007.09.075 |
| [39] | Zhang CZ, Li D, Yu HS, et al. Purification and characterization of piceid-β-d-glucosidase from Aspergillus oryzae. Process Biochem, 2007, 42(1): 83-88. DOI:10.1016/j.procbio.2006.07.019 |
| [40] | Ko JA, Park JY, Kwon HJ, et al. Purification and functional characterization of the first stilbene glucoside-specific β-glucosidase isolated from Lactobacillus kimchi. Enzyme Microb Technol, 2014, 67: 59-66. DOI:10.1016/j.enzmictec.2014.09.001 |
| [41] | 冯薇, 胡小妍, 马明娜, 等. 产β-葡萄糖苷酶细菌的筛选及转化白藜芦醇的研究. 生物技术通报, 2017, 33(11): 130-135. Feng W, Hu XY, Ma MN, et al. The screening of β-glycosidase-producing strain and the transforming of resveratrol. Biotechnol Bull, 2017, 33(11): 130-135 (in Chinese). |
| [42] | Wang CQ, Liu XL, Zhang ML, et al. Efficient enzyme-assisted extraction and conversion of polydatin to resveratrol from Polygonum cuspidatum using thermostable cellulase and immobilized β-glucosidase. Front Microbiol, 2019, 10: 445. DOI:10.3389/fmicb.2019.00445 |
