
 , 冯铁山1, 龚娇1, 刘春1,2, 夏庆友1,2
, 冯铁山1, 龚娇1, 刘春1,2, 夏庆友1,21 西南大学 家蚕基因组生物学国家重点实验室, 重庆 400715;
2 西南大学 重庆市蚕丝纤维新材料工程技术研究中心, 重庆 400715
基金项目:国家自然科学基金(No. 31372380), 重庆基础和前沿研究计划(No. CSTC2017JCYJAX0090), 中央高校基础研究基金(No.XDJK2017C075)资助
摘要: 苏云金芽孢杆菌Bacillus thuringiensis生产的晶体毒素被广泛用作农林害虫的杀虫剂。鳞翅目昆虫受体蛋白是阐明其与晶体毒素相互作用的重要模式。文中纯化了苏云金芽孢杆菌的晶体毒素蛋白, 质谱鉴定为Cry1Ac毒素, 然后重组表达家蚕氨肽酶N (BmAPN6)和类钙粘蛋白(CaLP)结合结构域。利用免疫共沉淀、Far-Western印迹和酶联免疫吸附试验, 证明Cry1Ac毒素蛋白和BmAPN6之间的相互作用。在Sf9细胞中, 对Cry1Ac毒素的细胞毒活性分析, 表明BmAPN6参与Cry1Ac毒素诱导的细胞形态异常和裂解死亡。文中也利用相同的方法, 对钙粘蛋白的3个结合位点CR7、CR11和CR12进行相互作用分析, 结果表明3个重复结构域是CaLP的Cry1Ac结合位点。上述结果表明, BmAPN6和CaLP可作为Cry1Ac毒素致病的功能性受体, 为进一步揭示晶体毒素的致病机制和基因编辑增强家蚕抗病性提供了研究靶标。
关键词: 氨肽酶 钙粘蛋白 晶体毒素 家蚕
Interaction of Bombyx mori aminopeptidase N and cadherin-like protein with Bacillus thuringiensis Cry1Ac toxin
Ping Lin1, Tingcai Cheng1,2

 , Tieshan Feng1, Jiao Gong1, Chun Liu1,2, Qingyou Xia1,2
, Tieshan Feng1, Jiao Gong1, Chun Liu1,2, Qingyou Xia1,2 1 State Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing 400715, China;
2 Chongqing Engineering and Technology Research Center for Novel Silk Materials, Southwest University, Chongqing 400715, China
Received: February 9, 2018; Accepted: May 10, 2018
Supported by: National Natural Science Foundation of China (No. 31372380), Chongqing Fundamental and Advanced Research Projects (No. CSTC2017JCYJAX0090), Fundamental Research Funds for the Central Universities (No. XDJK2017C075)
Corresponding author:Tingcai Cheng. Tel: +86-23-68251987; E-mail: chengtc@swu.edu.cn
Abstract: Bacillus thuringiensis (Bt) produces Cry toxins that are widely used as insecticides in agriculture and forestry. Receptors are important to elucidate the mode of interaction with Cry toxins and toxicity in lepidopteran insects. Here, we purified the Cry toxin from Bt and identified this toxin by flight mass spectrometry as Cry1Ac, and then recombinantly expressed aminopeptidase N (BmAPN6) and repeat domains of cadherin-like protein (CaLP) of B. mori. Using co-immunoprecipitation (co-IP), Far-Western blotting, and enzyme-linked immunosorbent assays (ELISAs), we identified the interaction between Cry1Ac and BmAPN6. Furthermore, analysis of the cytotoxic activity of Cry1Ac toxin in Sf9 cells showed that BmAPN6 directly interacted with Cry1Ac toxin to induce morphological aberrations and cell lysis. We also used co-IP, Far-Western blotting and ELISAs to analyze the interactions of Cry1Ac with three binding sites corresponding to cadherin repeat (CR) 7 CR11, and CR12 of CaLP. Notably, the three repeat domains were essential Cry1Ac binding components in CaLP. These results indicated that BmAPN6 and CaLP served as a functional receptor involved in Bt Cry1Ac toxin pathogenicity. These findings represent an important advancement in our understanding of the mechanisms of Cry1Ac toxicity and provide promising candidate targets for gene editing to enhance resistance to pathogens and increase the economic value of B. mori.
Keywords: aminopeptidase N cadherin-like protein Cry toxin Bombyx mori
Bacillus thuringiensis (Bt) is a Gram-positive bacterium that produces crystal proteins (Cry toxins) during sporulation. Cry toxins are encoded by different Cry genes located on plasmids in most Bt strains[1]. Because of the specific activities of Cry toxins against insect species, Cry toxins have been extensively used as biological pesticides for farming and forestry pests. A generally accepted mechanism is that Cry protoxins are activated in the larval alkaline midgut environment, and the activated toxins then bind with receptors on the midgut epithelial membrane to destroy midgut tissue and lead to larval death, likely through septicemia[2]. Receptors in the epithelial membrane play crucial roles in forming toxin oligomers to embed and lyse cells or trigger signaling cascades. To date, many types of receptors, including cadherin-like protein (CaLP)[3], aminopeptidase N (APN)[4], alkaline phosphatase (ALP)[5], and ATP-dependent binding cassette (ABC) transporter[6], have been well characterized in Lepidoptera species.
Manduca sexta CaLP was the first Cry1A receptor identified following purification from the midgut epithelium[3] and was cloned as the Bt-R1 gene[7] in Lepidoptera. Subsequently, CaLPs as Cry1A receptors were widely identified in several Lepidoptera insects, including Bombyx mori[8], Heliothis virescens[9], Pectinophora gossypiella[10], Helicoverpa armigera[11] and Ostrinia nubilalis[12]. Lepidoptera CaLPs contain 9–12 cadherin repeats (CRs), which are necessary for Cry binding, particularly for CRs close to the transmembrane region[13]. CR7, CR11 and CR12 of CaLP interacting with Cry1A-type toxins are essential for their toxicity in M. sexta[14-15]. APN M. sexta was the first molecule that was tentatively identified as a Cry toxin-binding protein[4]. A series of lepidopteran APNs were shown to bind to Cry1Ac in H. virescens, Plutella xylostella and Trichoplusia ni[16-18]. Moreover, the absence, mutation or knockdown of APNs is associated with resistance to Cry toxin in Lepidoptera insects[19-21].
Unlike the above receptors, ABC transporters were found to be responsible for insect resistance against Cry toxins using forward genetics approaches, such as linkage mapping and position cloning analysis, in Lepidoptera[6, 22-23]. A silkworm mutant with an amino acid insertion in ABC transporter C2 (BmABCC2) was resistant to Cry1A toxin and could be susceptible to Cry1A when transformed with a wild-type BmABCC2 gene[23]. Overexpression of BmABCC2 in Sf9 cells imparts susceptibility to Cry1A, Cry1F and Cry8Ca toxins[24]. Moreover, defects in the 12th transmembrane (TM) domain of ABCC2 confer Cry1Ac resistance in P. xylostella by a 30-bp deletion in the gene encoding the protein[6]; however, overexpression of Px-abcc2 in the Drosophila midgut conferred high susceptibility to both Cry1Ac protoxin and trypsin-activated toxin[25]. Recently, ABCC2 was applied as a causal factor in insecticidal resistance owing to its important binding with Cry1A toxins, similar to CaLPs.
As a uniquely economic insect, B. mori is also a useful model host for studies on insecticidal resistance. Several receptors have been identified in B. mori, including a GaLP[8], an ABC transporter [23] and four APNs[26-27].B. mori GaLP, also known as BtR175, binds Cry1Aa toxin on the last CR domain adjacent to the membrane[28]. All four APNs bind Cry1Aa and/or Cry1Ab toxins[27]. In a genome-wide analysis, researchers annotated 16 APNs in the silkworm genome[29]. However, it is unclear whether other members are also functional receptors and whether additional CR domains of CaLP can facilitate binding with Cry1Ac toxin.
B. thuringiensis subspecies kurstaki strain HD73, toxic to lepidopteran larvae, contains only one endotoxin gene, the cry1Ac gene, which was found in the pHT73 plasmid[30]. Accordingly, in this study, we cloned and expressed recombinant BmAPN6 and three CR domains (CR7, CR11 and CR12) of CaLP. We then evaluated the susceptibility of Sf9 cells expressing BmAPN6 to Cry1Ac and used Far-Western blotting, co-immunoprecipitation, pull-down assays, and enzyme-linked immunosorbent assay (ELISA) binding assays to assess the interactions among BmAPN6, the three CR domains, and Cry1Ac toxin. Furthermore, based on mode of action of Bt Cry toxin in the lepidopteran[31], we propose a schematic model for the process by Bt Cry1Ac interacting with CaLP and BmAPN6. Specifically, Bt Cry1Ac binds to the high-abundance BmAPN6 receptor, allowing the toxin to locate in close proximity to the membrane, which is followed by high-affinity binding to the CaLP receptor by the cadherin fragments CR7, CR11 and CR12, leading to disrupts homeostasis and material exchange throughout the insect body. In addition, Bt Cry toxins are known as the most successful microbial insecticide against different orders of insect, especially for insect pests, in agriculture. Our results reveal that Bt Cry1Ac interacts with BmAPN6 and CaLP makes them as good candidate for deployment with Bt Cry1Ac-resistant silkworm to enhance economic benefit of sericulture.
1 Materials and methods1.1 Purification of Cry proteins from BtParasporal crystal inclusions were purified from B. thuringiensis subsp. kurstaki strain HD73[30], which expressed the Cry1Ac toxin. Briefly, after 48 h of incubation at 37 ℃, the spore-crystal mixtures of Bt were centrifuged at 8 000×g for 20 min. The pellet was washed three times with 1 mol/L ice-cold NaCl at 8 000×g for 20 min and then washed three times with distilled water. The pellet was suspended in sodium carbonate buffer containing 50 mmol/L Na2CO3 (pH 9.5) and 10 mmol/L dithiothreitol. After incubation, the samples were centrifuged at 8 000×g at 4 ℃ to obtain supernatants. The supernatants were adjusted to pH 4.5 with 4 mol/L CH3COONa-CH3COOH buffer overnight and were then centrifuged12 000×g for 20 min at 4 ℃. The pellets were washed three times with distilled water, solubilized in 1 mL of 10 mmol/L NaOH, and incubated for 4 h at 4 ℃. After centrifugation at 12 000×g for 10 min, the supernatant containing the solubilized parasporal crystals was retained. The solubilized parasporal crystal proteins were activated by treatment with trypsin for 6 h using 1 μg trypsin per 20 μg protein[32]. The purified protein was then added to 1/10 (V/V) 4 mol/L CH3COONa- CH3COOH buffer and centrifuged for 10 min at 12 000×g as described above. The protein concentration was determined by the Bradford method using bovine serum albumin (BSA) as a standard. The protein profiles were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels with Coomassie brilliant blue staining.
1.2 Expression vector construction and recombinant protein expressionBmAPN6 (BGIBMGA008061) was cloned into the BamHⅠ/NotⅠ sites of the vector pGEX-4T-1 (GE Healthcare Life Sciences) with a GST tag on the N-terminal end. The recombinant vector was transformed into Escherichia coli BL21 (DE3) to induce the expression of GST-tagged BmAPN6 (GST-BmAPN6). Next, the recombinant protein was induced by adding isopropyl-β-D-thiogalactoside (IPTG) to a final concentration of 0.1 mmol/L, and cells were grown for 5 h at 37 ℃. Cells were collected by centrifugation and resuspended in phosphate-buffered saline (PBS: 140 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 1.8 mmol/L KH2PO4, pH 7.3). After lysing by sonication, Triton X-100 was added to a final concentration of 1%, and samples were mixed gently at room temperature (25 ℃) for 30 min to solubilize proteins. The crude extract was then centrifuged at 10 000×g for 5 min at 4 ℃. GST-BmAPN6 was purified by GSTrap FF (GE Healthcare), according to the manufacturer's instructions. Cadherin fragments encoding repeats CR7 (M811–A927), CR11 (L1257–T1398), and CR12 (N1368–G1484) of cadherin-like membrane protein BtR175 (NM_001044217) were cloned into pET-28a expression vectors with a 6×His tag, as previously described[33]. The His-CR7, His-CR11 and His-CR12 proteins were purified by affinity chromatography using Ni-NTA (GE Healthcare). The purified proteins were used for subsequent experiments.
1.3 Cell transfectionThe transfection plasmid was constructed by cloning the open reading frame (ORF) encoding BmAPN6 into the pSL1180[hr3-BmAct4-DsRed-SV40] plasmid between the BamHⅠ and NotⅠ restriction sites, yielding the plasmid pSL1180[hr3-BmAct4-BmAPN6-SV40]. As described in a previous study[33], Sf9 cells (Invitrogen, Carlsbad, CA, USA) were cultivated in Grace medium (Gibco) supplemented with 10% fetal bovine serum, 0.35 g/L NaHCO3, and antibiotic-antimycotic (HyClone) at 27 ℃. Cell transfection with the constructed plasmid was performed using Cellfectin Ⅱ Reagent (Invitrogen), according to the manufacturer's instruction (Protocol Pub. No. MAN0007821). For immunofluorescence analysis, cells were collected and washed with PBS and incubated with anti-APN6 antibody from mice at room temperature for 1 h. After three washes with PBS, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (1:500) at room temperature for 1 h. Finally, following three washes with PBS, cells were mounted in buffered glycerol and observed under a fluorescence microscope. Primers (BmAPN6 F: 5?-CCTAACATCGGCTGACCACCCA-3?; R: 5?-AT CCCCTCCAAATACCCCTCTT-3?) were used to test the expression of BmAPN6 by reverse transcription polymerase chain reaction (RT-PCR). The ribosomal_30 gene (accession number: AF400225.1; F: 5?-TGGCTCGTGCTGGTAAGG-3?; R: 5?-CTGCGTCCGAATGTCTGG-3?) was used as internal control.
1.4 Cytotoxicity assaySf9 cells were harvested and transferred to 48-well tissue culture dishes (Costar) at 1×105 cells per well. After the cells settled, they were transfected with pSL1180[hr3-BmAct4-BmAPN6-SV40]. After 48 h, the cell layer was washed gently with PBS three times and overlaid with activated Cry1Ac toxin (50 μg/mL in PBS). The cells were incubated with activated Cry1Ac toxin for 6 h and were then observed under an Olympus TH4-200 microscope. The total number of cells per field was counted. Final values represent an average of 50 fields, which were randomly selected for each treatment. This assay measured the amount of stable cytosolic lactate dehydrogenase (LDH) released from the lysed cells using a coupled enzymatic assay[34]. Briefly, the culture medium was replaced with 200 μL HEPES-buffered Dulbecco's modified Eagle's medium (DMEM) containing 1% bovine serum albumin (BSA) and various concentrations of activated Cry1Ac. The cells were incubated at 37 ℃ for 1 h and centrifuged at 250×g for 10 min. A 100 μL aliquot of the supernatant was transferred to another microplate. The LDH activity in the supernatant was measured using an LDH Cytotoxicity Detection Kit (TaKaRa Biomedicals, Shiga, Japan) following the manufacturer's instructions. The amount of released LDH activity was indicated as a proportion of the total LDH activity, which was measured by disrupting untreated cells with 1% Triton X-100. Background leakage of LDH activity, which occurred without Cry protein during the incubation, was separately measured and subtracted from all the data, including the total LDH activity. All experiments were performed in triplicate.
1.5 Western blottingPlasma membrane proteins were collected when Sf9 cells were transfected with BmAPN6 48 h later, as previously described[35]. Briefly, cells were harvested, resuspended in extraction buffer, and incubated on ice under a microscope for complete lysis. Cell lysates were combined with sucrose buffer and centrifuged at 5 000×g for 10 min. The supernatants were then subjected to ultracentrifugation at 100 000×g for 60 min at 4 ℃. High-speed pellets were solubilized in Triton-X lysis buffer containing phosphatase inhibitor (Pierce) and protease inhibitor cocktail (Roche). Total membrane proteins were resolved by SDS-PAGE on 10% gels. After the proteins were transferred to polyvinylidene difluoride (PVDF) membranes, they were immunoblotted with anti-BmAPN6 antibodies or anti-tubulin monoclonal antibodies (Sigma- Aldrich, St. Louis, MO, USA) following standard procedures.
1.6 Far-Western blottingFar-Western blotting was performed as previously described[36]. Briefly, the trypsin- activated Cry1Ac toxin was electrophoresed by SDS-PAGE on 12% (W/V) gels and transferred to PVDF membranes at 100 V for 1 h at 4 ℃. The transferred membrane was blocked with 5% milk in PBST for 1 h at room temperature and then incubated with recombinant BmAPN6, CR7, CR11, CR12, GST and BSA proteins in protein-binding buffer overnight at 4 ℃. After washing the membrane with PBST three times, the blots were incubated with anti-GST or anti-His antibodies (1:8 000) targeting the recombinant proteins for 1 h in 3% milk in PBST and then incubated with secondary antibodies (1:10 000) for 1 h. Chemiluminescent detection was performed using an ECL kit according to the manufacturer's instructions.
1.7 Co-immunoprecipitation (Co-IP)One hundred micrograms of BmAPN6 protein with GST tag at N terminal was incubated with anti-GST antibodies for 1 h at room temperature, and the complexes were then bound to Protein A magnetic beads (Thermo Fisher Scientific) at room temperature, according to the manufacturer's instructions. BS3 (Thermo Fisher Scientific) was used for crosslinking. After washing, the beads were incubated with trypsin-activated Cry1Ac protein (100 μg) overnight at 4 ℃. The complexes were eluted after washing and were separated by SDS-PAGE on 10% gels stained with silver staining.
1.8 Pull-down assaysHis pull-down experiments were performed using a Pierce Protein Interaction Pull-Down Kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Briefly, purified CR7, CR11 and CR12 were added to a Pierce Spin Column and incubated at 4 ℃ for at least 30 min with gentle rocking on a rotating platform. After five washes using wash solution, trypsin-activated Cry1Ac was added to the Pierce Spin Column and incubated at 4 ℃ for at least 1 h. After another five washes, the spin columns were incubated with elution buffer for 5 min and centrifuged at 1 250 × g for 30–60 s to collect the sample.
1.9 ELISA binding assaysOne microgram of trypsin-activated Cry1Ac was added to ELISA plates in 100 μL PBS/well overnight at 4 ℃. The plates were washed three times with PBS and then blocked with 200 μL/well PBS-M for 2 h at 37 ℃. After three washes with PBS, the purified proteins (BmAPN6, CR7, CR11 and CR12) were used for incubation. The unbound proteins were removed by three washes with PBS-T and three washes with PBS. The bound proteins were detected using rabbit anti-BmAPN6 antibody or anti-His tag antibody and anti-rabbit or anti-mouse antibodies conjugated with horseradish peroxidase. Finally, ortho-phenylenediamine (Sigma) plus H2O2 was used as the substrate for detection. The reaction was stopped by adding 50 μL of 1 mmol/L H2SO4, and the absorbance was measured at 490 nm using an ELISA microplate reader.
1.10 Statistical analysisStatistical analysis was performed with GraphPad (GraphPad Software, La Jolla, CA, USA) using Student's t-tests. Differences with P values of less than 0.05 were considered significant.
2 Results2.1 Purification of Bt Cry toxin, BmAPN6, and CaLP fragmentsTo investigate the mechanisms of Cry toxins in the lepidopteran insect B. mori, we first purified Bt Cry1Ac (Fig. 1A, lanes 1, 2). Because Bt protoxin is not toxic to Lepidoptera insects, Cry protoxins are processed by midgut proteases for proteolytic removal of the C-terminal half and N-terminal peptide, which is important for toxicity[37]. The purified Bt Cry was digested with trypsin to obtain the activated Cry toxin (Fig. 1A, lane 3). The results of matrix-assisted laser desorption/ionization-time of flight mass spectrometry showed that nine peptides matched insecticidal Cry1Ac (gi|87298907) in this band (Table 1). To investigate the specificity of Bt Cry1Ac toxins for B. mori receptors, the B. mori midgut receptor BmAPN6 with the GST tag (Fig. 1B) and three cadherin fragments corresponding to CR7 (Fig. 1Ca), CR11 (Fig. 1Cb) and CR12 (Fig. 1Cc) were expressed and purified.
 |
| Figure 1 Purification of Cry1Ac toxin and recombinant proteins. (A) SDS-PAGE analysis of the purification of Bt Cry1Ac from Bt (lanes 1, 2) and trypsin-activated Cry1Ac (lane 3). (B) Recombinant GST-BmAPN6 protein purified from the Escherichia coli expression system by GSTrap FF purification. (C) CR7, CR11 and CR12 fragments of BtR-175 were expressed as recombinant proteins with His-tags and purified by affinity chromatography using Ni-NTA |
| 图选项 |
Table 1 MALDI-TOF/TOF analysis of the purified Bt Cry1Ac
| Accession number | Protein description | Matched peptides |
| gi|87298907 | Insecticidal crystal protein (Bt Cry1Ac) | APMFSWIHR |
| APTFSWQHR | ||
| GYIEVPIHFPSTSTR | ||
| IVAQLGQGVYR | ||
| LIGNYTDYAVR | ||
| SGTVDSLDEIPPQNNNVPPR | ||
| VWGPDSRDWVR | ||
| WGFDAATINSR | ||
| WYNTGLER |
表选项
2.2 Binding assays of Cry1Ac with BmAPN6To test whether BmAPN6 interacted with Cry1Ac, we performed Co-IP, Far-Western blotting, and ELISA. Far-Western blotting analysis showed that positive bands were observed only when the trypsin-activated Cry1Ac/BmAPN6 complexes were present, indicating that activated Cry1Ac toxin could bind to silkworm BmAPN6 (Fig. 2A). As shown in Fig. 2B, the Co-IP assay demonstrated that trypsin-activated Cry1Ac was capable of interacting with BmAPN6. Furthermore, ELISA binding saturation assays showed that trypsin-activated Cry1Ac bound to BmAPN6 (Fig. 2C), which confirmed the interaction between Bt Cry1Ac toxin and BmAPN6.
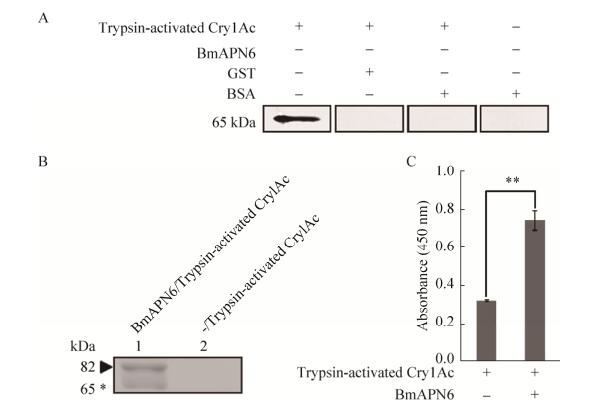 |
| Figure 2 Binding assays of trypsin-activated Cry1Ac and BmAPN6. (A) Far-Western blotting analysis of trypsin-activated Cry1Ac and BmAPN6. Trypsin-activated Cry1Ac was separated by SDS-PAGE on 12% (W/V) gels and transferred to PVDF membranes for Far-Western blotting analysis. The recombinant BmAPN6 (Lane 1), GST (Lane 2) and BSA (Lane 3) proteins were incubated with the membranes before adding anti-GST antibodies. The membranes were used for direct immunoblotting with anti-GST antibodies (Lane 4). Positive bands were observed only when trypsin-activated Cry1Ac/BmAPN6 complexes were present. (B) Co-IP assay for trypsin-activated Cry1Ac and BmAPN6. Lane 1 shows trypsin-activated Cry1Ac incubated with BmAPN6, which was immunoprecipitated with anti-GST antibodies. Lane 2 shows trypsin-activated Cry1Ac immunoprecipitated with anti-GST antibodies as a control. The complexes were separated by SDS-PAGE on 10% gels stained with silver staining. Triangle: BmAPN6; asterisk: trypsin-activated Cry1Ac. (C) ELISA binding saturation assays of trypsin-activated Cry1Ac and BmAPN6. Data were analyzed using GraphPad Prism software, and data are shown as mean SEM of three experiments. Statistically significant differences from the control samples are indicated as **P < 0.01 |
| 图选项 |
2.3 Cry1Ac toxin induced Sf9 cell death via BmAPN6To ascertain whether BmAPN6 was involved in Cry1Ac toxin pathogenicity, the cytotoxic activity of Cry1Ac toxin in Sf9 cells transfected with the BmAPN6 gene was tested. RT-PCR analysis showed that BmAPN6 was expression in Sf9 Cells (Fig. 3A). Furthermore, immunofluorescence was used to evaluate the location of BmAPN6 in cells (Fig. 3B). In addition, BmAPN6 was expressed in the membrane fractions of Sf9 cells as demonstrated by Western blotting (Fig. 3C), indicating that BmAPN6 may be as a membrane receptor. Separately, the incubation of live, viable Sf9 cells expressing BmAPN6 with trypsin-activated Cry1Ac toxin induced distinct cytological changes, including swelling and lysis, which led to cell death (Fig. 3D-d), suggesting that expressed BmAPN6 directly interacted with Cry1Ac toxin to induce morphological aberrations and cell lysis. The number of viable cells after trypsin-activated Cry1Ac toxin administration was counted in 50 different optical fields. The results showed that the numbers of viable Sf9 cells expressing BmAPN6 after treatment with trypsin- activated Cry1Ac toxin were reduced (Fig. 3E). Cytotoxicity assays based on the extracellular release of LDH activity indicated that trypsin-activated Cry1Ac toxin caused a 16.4% increase in the release of LDH activity from BmAPN6-transfected Sf9 cells into the culture medium (Fig. 3F). Together with the results of the binding assay in Fig. 2, these findings indicated that BmAPN6 served as a functional receptor involved in Bt Cry1Ac toxin pathogenicity.
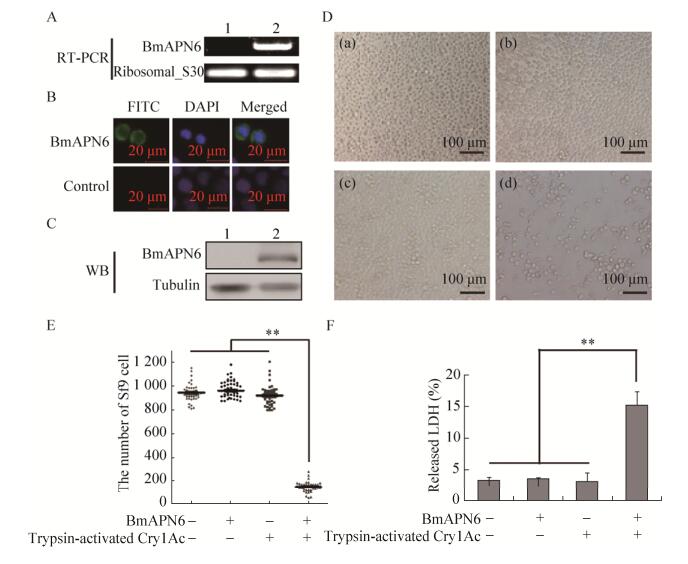 |
| Figure 3 Trypsin-activated Cry1Ac induced cell death in Sf9 cells expressing BmAPN6. (A) RT-PCR analysis of Sf9 cells expressing BmAPN6. Total RNA was extracted from Sf9 cells at 48 h after transfection with the psl1180[hr3-BmAct4-BmAPN6-SV40] vector. Lane 1 shows native Sf9 cells as a control. Lane 2 shows BmAPN6 expressed by Sf9 cells; the gene encoding ribosomal protein S30 was used as an internal control for RT-PCR. (B) The localization of BmAPN6 in Sf9 cells was observed by microscopy. (C) Western blotting analysis of Sf9 cells expressing BmAPN6. Membrane proteins were extracted from Sf9 cells at 48 h after transfection with the psl1180[hr3-BmAct4-BmAPN6-SV40] vector. Tubulin was used as an internal control for Western blotting. (D) Sf9 cells exposed to trypsin-activated Cry1Ac toxin in the presence or absence of BmAPN6. Photomicrographs of healthy Sf9 cells (a), BmAPN6-expressing Sf9 cells (b), healthy Sf9 cells treated with trypsin-activated Cry1Ac (c), and BmAPN6-expressing Sf9 cells overlaid with trypsin-activated Cry1Ac (d) are shown. BmAPN6-expressing cells incubated with trypsin-activated Cry1Ac showed swelling and lysis. (E) The number of BmAPN6-expressing Sf9 cells displaying morphological aberrations after incubation with trypsin-activated Cry1Ac. (F) Trypsin-activated Cry1Ac-induced cell death was measured based on the extracellular release of LDH activity after incubation for 6 h. Data are shown as mean SEM of three experiments. Statistically significant differences from the control samples are indicated as **P < 0.01 |
| 图选项 |
2.4 Interaction of Cry1Ac with cadherin fragments of CaLPBt Cry1A(a) toxin binds to a 175 kDa glycoprotein (BtR-175) on the microvillus membranes of columnar cells in the B. mori midgut and causes lysis of the cells[8]. Using Escherichia coli, we previously cloned and produced three cadherin fragments corresponding to CR7, CR11 and CR12 of B. mori CaLP, corresponding to M. sexta Bt-R1. We performed His pull-down assays of trypsin- activated Cry1Ac toxin with cadherin fragment proteins, indicating that activated Cry1Ac toxin bound to CR7, CR11 and CR12 (Fig. 4A). As shown in Fig. 4B, Far-Western blotting showed the positive bands that reacted with the anti-His antibody only when the trypsin-activated Cry1Ac/CR7, trypsin- activated Cry1Ac/CR11, and trypsin-activated Cry1Ac/CR12 complexes were present. Furthermore, ELISA binding assays confirmed that trypsin- activated Cry1Ac bound to CR7, CR11 and CR12. These results showed that Bt Cry1Ac toxin interacted with B. mori CaLP by binding to CRs.
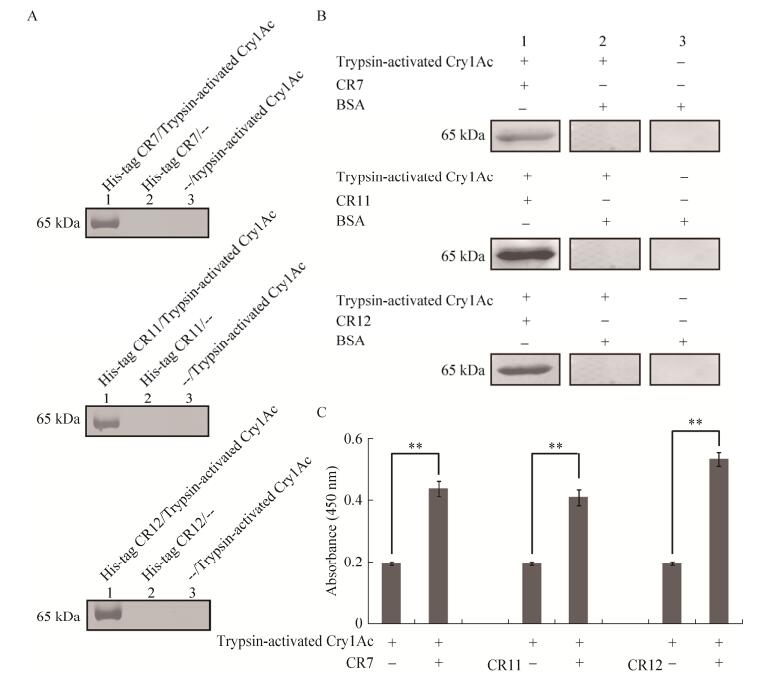 |
| Figure 4 Interaction of trypsin-activated Cry1Ac with CR domains of CaLP. (A) His-tag pull-down assays for CaLP fragments with trypsin-activated Cry1Ac. Lane 1 shows His-CR7, His-CR11 and His-CR12 incubated with trypsin-activated Cry1Ac and purified with Ni-NTA beads. The bands represent the trypsin-activated Cry1Ac protein, which bound to His-CR7, His-CR11 and His-CR12, respectively. Lane 2 shows His-CR7, His-CR11 and His-CR12 incubated with Ni-NTA beads as a negative control. Lane 3 represents another negative in which Ni-NTA beads alone were incubated with trypsin-activated Cry1Ac protein. (B) Far-Western blotting analysis of Cry1Ac-CaLP interactions. Trypsin-activated Cry1Ac was separated by SDS-PAGE using 12% (W/V) gels and transferred to PVDF membranes. The recombinant His-CR7, His-CR11 and His-CR12 proteins (Lane 1) were incubated with membranes. The membrane was either incubated with BSA (lane 2) or directly immunoblotted (Lane 3) as a negative control. Positive bands were observed with anti-His tag when trypsin-activated Cry1Ac/CR7, trypsin-activated Cry1Ac/CR11, and trypsin-activated Cry1Ac/CR12 complexes were present. (C) ELISA binding saturation assays of trypsin-activated Cry1Ac and CaLP fragments. Data are shown as mean SEM of three experiments. Statistically significant differences from the control samples are indicated as **P < 0.01 |
| 图选项 |
3 DiscussionProtoxins produced by bacteria are known to be activated by proteolytic cleavage in the host alkaline midgut environment. Activated toxins bind with receptors on the midgut epithelial membrane undergoing oligomerization to form pores and lyse cells, thereby leading to cell death. Thus, receptors in host midgut brush border membrane vesicles play crucial roles in the pathogenicity of insect pathogens. In the study, we used several methods to show that Cry1Ac could interact with 1 BmAPN6, a member of the APN gene family (using Far-Western blotting, Co-IP and ELISA); and 2 CR domains from B. mori CaLP (using pull-down assays, Far-Western blotting and ELISA). APNs are extensively studied putative receptors isolated from many lepidopteran insects[38]. Four APNs have been shown to interact with toxins[26-27]. BmAPN2, BmAPN3 and BmAPN4, which have molecular masses ranging from 90 to 110 kDa, can interact with Cry1Ab but not with Cry1Aa[27]. Native BmAPN1 and CaLP had high-affinity binding with Cry1Aa[39]. Our results showed that one member of the APN gene family from B. mori, BmAPN6, interacted with Cry1Ac. Furthermore, BmAPN6 was found to function as a receptor for Cry1Ac in Sf9 cells, suggesting that APNs may be crucial receptors involved in the mechanisms underlying toxicity. Three CRs (CR7, CR11 and CR12) from silkworm CaLP can interact with Cry1Ac, similar to three corresponding Cry1Ab- binding domains from M. sexta Bt-R1[13-15, 40]. Moreover, a PC toxin purified from B. bombysepticus showed obvious interactions with CR7 and CR12, barely detectable interactions with CR11, and binding with BmAPN4 and BmAPN5[33, 41-42]. Thus, these data suggested that multiple receptors play crucial roles in toxin binding. Based on previous research of Bt Cry toxin mode of action[31]. We propose a schematic model for the process by Bt Cry1Ac interacting with CaLP and BmAPN6 (Fig. 5). Specifically, Bt Cry1Ac binds to the high-abundance BmAPN6 receptor, allowing the toxin to locate in close proximity to the membrane, which is followed by high-affinity binding to the CaLP receptor by the cadherin fragments CR7, CR11 and CR12, leading to disrupts homeostasis and material exchange throughout the insect body (Fig. 5). APN is a multiple gene family. Although different members had been demonstrated or verified to bind different type Cry toxins, systematic comparison of interaction between APN members and several Cry toxins was still lacking, which need to be investigated.
 |
| Figure 5 A schematic representation of the model for Bt Cry1Ac causing damage to the silkworm via BmAPN6 and CaLP. Bt Cry1Ac can be digested by gut proteases. The activated toxin binds the high-abundance BmAPN6 receptor, allowing the toxin to be located to cell membrane and the interaction with the CaLP by CR7, CR11 and CR12, leading to cell lysis and death |
| 图选项 |
Proteins that bind with or act as receptors for toxins are complex and varied rather than monotonic. This is a common characteristic of multigene families such as APNs and ABC transporters, or repeats domain-containing proteins such as CaLPs, and may be an effective strategy for continuous evolution of pathogens to overcome the defense systems of insect hosts. If even one receptor mutation results in loss of binding capacity, toxins can utilize other binding receptors or members of the same family. Thus, single gene mutations in host insects may not confer complete resistance. For example, in H. virescens, CaLP mutations account for 40%–80% of the Cry resistance phenotype[9], and ABCC2 can confer a higher level of resistance than CaLP[22]; however, none of these mutations confer absolute resistance. This can explain why toxins selectively utilize different families and repeat domain-containing proteins as receptors instead of some simple structural proteins encoded by single-copy genes. Another strategy is the rapidly evolutionary divergent toxin families. To date, more than 700 genes encoding Cry toxins have been identified from the soil bacterium Bt[43-44]. The toxic activities and binding receptors of Cry toxins differ, even among the same evolutionary subclass. For example, H. virescens CaLP functions as a receptor for Cry1A toxins, but not Cry1Fa toxin[45]. In H. armigera, APN1 interacts with Cry1Aa, Cry1Ab and Cry1Ac, whereas APN2 only interacts with Cry1Ac[46]. Recently, wild-type Cry1Ac was found to be rapidly evolved to variants by a phage-assisted continuous evolution (PACE) selection[47]. Evolved variants of Cry1Ac bind T. ni CaLP and kill Cry1Ac-resistant T. ni strains with about 335-fold more potency than wild-type Cry1Ac[47]. Thus, rapidly evolutionary toxins should provide more targets for insect control.
Insect toxin resistance is disadvantageous to agricultural and forestry pest control, however, for the economically valuable insect B. mori, such resistance may be useful for sericulture. In Lepidoptera insects, toxin resistance of pest larvae has been introduced using several mutant models, such as deletion mutations, mRNA mis-splicing, mutations in regulatory regions, and protein mislocalization. For example, a Cry1Ac-resistant strain of H. armigera, derived from selection with toxins, is a deletion mutant of the APN1 gene[21]. A mutant CaLP of H. armigera was mislocalized in the endoplasmic reticulum instead of the plasma membrane, leading to resistance to Cry1Ac toxin[48]. In the silkworm, a Cas9/gRNA-induced mutant of CaLP increased the resistance to PC toxin and B. bombysepticus[33, 49]. Although the molecular mechanism is still unclear, a single amino acid insertion in ABCC2 indeed enhances resistance to Cry toxins in the mutant silkworm[23]. Thus, elucidation of the clear biological functions of receptors, such as APNs, CaLP and ABC transporters, will provide promising candidate targets for the genetic control of B. mori in order to achieve enhancement of resistance to pathogens and virulence factors.
REFERENCES
| [1] | Berry C, Crickmore N. Structural classification of insecticidal proteins-towards an in silico characterisation of novel toxins. J Invertebr Pathol, 2017, 142: 16-22. DOI:10.1016/j.jip.2016.07.015 |
| [2] | Bravo A, Gill SS, Soberón M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon, 2007, 49(4): 423-435. DOI:10.1016/j.toxicon.2006.11.022 |
| [3] | Vadlamudi RK, Ji TH, Bulla LA Jr. A specific binding protein from Manduca sexta for the insecticidal toxin of Bacillus thuringiensis subsp. berliner. J Biol Chem, 1993, 268(17): 12334-12340. |
| [4] | Knight PJK, Knowles BH, Ellar DJ. Molecular cloning of an insect aminopeptidase N that serves as a receptor for Bacillus thuringiensis CryIA(c) toxin. J Biol Chem, 1995, 270(30): 17765-17770. DOI:10.1074/jbc.270.30.17765 |
| [5] | McNall RJ, Adang MJ. Identification of novel Bacillus thuringiensis Cry1Ac binding proteins in Manduca sexta midgut through proteomic analysis. Insect Biochem Mol Biol, 2003, 33(10): 999-1010. DOI:10.1016/S0965-1748(03)00114-0 |
| [6] | Baxter SW, Badenes-Pérez FR, Morrison A, et al. Parallel evolution of Bacillus thuringiensis toxin resistance in lepidoptera. Genetics, 2011, 189(2): 675-679. DOI:10.1534/genetics.111.130971 |
| [7] | Vadlamudi RK, Weber E, Ji I, et al. Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J Biol Chem, 1995, 270(10): 5490-5494. DOI:10.1074/jbc.270.10.5490 |
| [8] | Nagamatsu Y, Toda S, Koike T, et al. Cloning, sequencing, and expression of the Bombyx mori receptor for Bacillus thuringiensis insecticidal Cry1A(a) toxin. Biosci Biotechnol Biochem, 1998, 62(4): 727-734. DOI:10.1271/bbb.62.727 |
| [9] | Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens. Science, 2001, 293(5531): 857-860. DOI:10.1126/science.1060949 |
| [10] | Morin S, Biggs RW, Sisterson MS, et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci USA, 2003, 100(9): 5004-5009. DOI:10.1073/pnas.0831036100 |
| [11] | Wang G, Wu K, Liang G, et al. Gene cloning and expression of cadherin in midgut of Helicoverpa armigera and its Cry1A binding region. Sci China C Life Sci, 2005, 48(4): 346-356. DOI:10.1360/03yc0273 |
| [12] | Flannagan RD, Yu CG, Mathis JP, et al. Identification, cloning and expression of a Cry1Ab cadherin receptor from European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae). Insect Biochem Mol Biol, 2005, 35(1): 33-40. DOI:10.1016/j.ibmb.2004.10.001 |
| [13] | Hua G, Jurat-Fuentes JL, Adang MJ. Bt-R1a extracellular cadherin repeat 12 mediates Bacillus thuringiensis Cry1Ab binding and cytotoxicity. J Biol Chem, 2004, 279(27): 28051-28056. DOI:10.1074/jbc.M400237200 |
| [14] | Gomez I, Miranda-Rios J, Rudi o-Pinera E, et al. Hydropathic complementarity determines interaction of epitope 869HITDTNNK876 in Manduca sexta Bt-R1 receptor with loop 2 of domain Ⅱ of Bacillus thuringiensis Cry1A toxins. J Biol Chem, 2002, 277(33): 30137-30143. DOI:10.1074/jbc.M203121200 |
| [15] | Gómez I, Dean DH, Bravo A, et al. Molecular basis for Bacillus thuringiensis Cry1Ab toxin specificity: two structural determinants in the Manduca sexta Bt-R1 receptor interact with loops α-8 and 2 in domain Ⅱ of Cy1Ab toxin. Biochemistry, 2003, 42(35): 10482-10489. DOI:10.1021/bi034440p |
| [16] | Gill SS, Cowles EA, Francis V. Identification, isolation, and cloning of a Bacillus thuringiensis Cry1Ac toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J Biol Chem, 1995, 270(45): 27277-27282. DOI:10.1074/jbc.270.45.27277 |
| [17] | Valaitis AP, Lee MK, Rajamohan F, et al. Brush border membrane aminopeptidase-N in the midgut of the gypsy moth serves as the receptor for the Cry1A(c) δ-endotoxin of Bacillus thuringiensis. Insect Biochem Mol Biol, 1995, 25(10): 1143-1151. DOI:10.1016/0965-1748(95)00050-X |
| [18] | Lee MK, You TH, Young BA, et al. Aminopeptidase N purified from gypsy moth brush border membrane vesicles is a specific receptor for Bacillus thuringiensis Cry1Ac toxin. Appl Environ Microbiol, 1996, 62(8): 2845-2849. |
| [19] | Herrero S, Gechev T, Bakker PL, et al. Bacillus thuringiensis Cry1Ca-resistant Spodoptera exigua lacks expression of one of four Aminopeptidase N genes. BMC Genomics, 2005, 6: 96. DOI:10.1186/1471-2164-6-96 |
| [20] | Sivakumar S, Rajagopal R, Venkatesh GR, et al. Knockdown of aminopeptidase-N from Helicoverpa armigera larvae and in transfected Sf21 cells by RNA interference reveals its functional interaction with Bacillus thuringiensis insecticidal protein Cry1Ac. J Biol Chem, 2007, 282(10): 7312-7319. DOI:10.1074/jbc.M607442200 |
| [21] | Zhang SP, Cheng HM, Gao YL, et al. Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mol Biol, 2009, 39(7): 421-429. DOI:10.1016/j.ibmb.2009.04.003 |
| [22] | Gahan LJ, Pauchet Y, Vogel H, et al. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet, 2010, 6(12): e1001248. DOI:10.1371/journal.pgen.1001248 |
| [23] | Atsumi S, Miyamoto K, Yamamoto K, et al. Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc Natl Acad Sci USA, 2012, 109(25): E1591-1598. DOI:10.1073/pnas.1120698109 |
| [24] | Tanaka S, Miyamoto K, Noda H, et al. The ATP-binding cassette transporter subfamily C member 2 in Bombyx mori larvae is a functional receptor for Cry toxins from Bacillus thuringiensis. FEBS J, 2013, 280(8): 1782-1794. DOI:10.1111/febs.2013.280.issue-8 |
| [25] | Stevens T, Song S, Bruning JB, et al. Expressing a moth abcc2 gene in transgenic Drosophila causes susceptibility to Bt Cry1Ac without requiring a cadherin-like protein receptor. Insect Biochem Mol Biol, 2017, 80: 61-70. DOI:10.1016/j.ibmb.2016.11.008 |
| [26] | Yaoi K, Nakanishi K, Kadotani T, et al. cDNA cloning and expression of Bacillus thuringiensis Cry1Aa toxin binding 120 kDa aminopeptidase N from Bombyx mori. Biochim Biophys Acta - Gene Struct Expr, 1999, 1444(1): 131-137. DOI:10.1016/S0167-4781(98)00250-4 |
| [27] | Nakanishi K, Yaoi K, Nagino Y, et al. Aminopeptidase N isoforms from the midgut of Bombyx mori and Plutella xylostella-their classification and the factors that determine their binding specificity to Bacillus thuringiensis Cry1A toxin. FEBS Lett, 2002, 519(1/3): 215-220. |
| [28] | Nagamatsu Y, Koike T, Sasaki K, et al. The cadherin-like protein is essential to specificity determination and cytotoxic action of the Bacillus thuringiensis insecticidal CryIAa toxin. FEBS Lett, 1999, 460(2): 385-390. DOI:10.1016/S0014-5793(99)01327-7 |
| [29] | Lin P, Cheng TC, Jin SK, et al. Structural, evolutionary and functional analysis of APN genes in the Lepidoptera Bombyx mori. Gene, 2014, 535(2): 303-311. DOI:10.1016/j.gene.2013.11.002 |
| [30] | Liu GM, Song L, Shu CL, et al. Complete genome sequence of Bacillus thuringiensis subsp. kurstaki strain HD73. Genome Announc, 2013, 1(2): e00080-13. |
| [31] | Bravo A, Likitvivatanavong S, Gill SS, et al. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem Mol Biol, 2011, 41(7): 423-431. DOI:10.1016/j.ibmb.2011.02.006 |
| [32] | Fiuza L, Nielsen-Leroux C, Goze E, et al. Binding of Bacillus thuringiensis Cry1 toxins to the midgut brush border membrane vesicles of Chilo suppressalis (Lepidoptera: Pyralidae): Evidence of Shared Binding Sites. Appl Environ Microbiol, 1996, 62(5): 1544-1549. |
| [33] | Lin P, Cheng TC, Jin SK, et al. PC, a novel oral insecticidal toxin from Bacillus bombysepticus Involved in Host Lethality via APN and BtR-175. Sci Rep, 2015, 5: 11101. DOI:10.1038/srep11101 |
| [34] | Tsuda Y, Nakatani F, Hashimoto K, et al. Cytotoxic activity of Bacillus thuringiensis Cry proteins on mammalian cells transfected with cadherin-like Cry receptor gene of Bombyx mori (silkworm). Biochem J, 2003, 369(3): 697-703. DOI:10.1042/bj20021401 |
| [35] | Wang F, Herzig C, Ozer D, et al. Tyrosine phosphorylation of scavenger receptor cysteine-rich WC1 is required for the WC1-mediated potentiation of TCR-induced T-cell proliferation. Eur J Immunol, 2009, 39(1): 254-266. DOI:10.1002/eji.200838472 |
| [36] | Wu YL, Li Q, Chen XZ. Detecting protein-protein interactions by Far Western blotting. Nat Protoc, 2007, 2(12): 3278-3284. DOI:10.1038/nprot.2007.459 |
| [37] | Bravo A, Sánchez J, Kouskoura T, et al. N-terminal activation is an essential early step in the mechanism of action of the Bacillus thuringiensis Cry1Ac insecticidal toxin. J Biol Chem, 2002, 277(27): 23985-23987. DOI:10.1074/jbc.C200263200 |
| [38] | Hughes AL. Evolutionary diversification of aminopeptidase N in Lepidoptera by conserved clade-specific amino acid residues. Mol Phylogenet Evol, 2014, 76: 127-133. DOI:10.1016/j.ympev.2014.03.014 |
| [39] | Jenkins JL, Dean DH. Binding specificity of Bacillus thuringiensis Cry1Aa for purified, native Bombyx mori aminopeptidase N and cadherin-like receptors. BMC Biochem, 2001, 2: 12. DOI:10.1186/1471-2091-2-12 |
| [40] | Gómez I, Oltean DI, Gill SS, et al. Mapping the epitope in cadherin-like receptors involved in Bacillus thuringiensis Cry1A toxin interaction using phage display. J Biol Chem, 2001, 276(31): 28906-28912. DOI:10.1074/jbc.M103007200 |
| [41] | Cheng TC, Lin P, Jin SK, et al. Complete genome sequence of Bacillus bombysepticus, a Pathogen Leading to Bombyx mori black chest septicemia. Genome Announc, 2014, 2(3): e00312-14. |
| [42] | Fu JF, Lin P, Feng TS, et al. Interaction of aminopeptidase (BmAPN5) and parasporal crystal (PC) toxin isolated from Bacillus bombysepticus. Chin J Biotech, 2017, 33(1): 90-98. 付剑锋, 林平, 冯铁山, 等. 家蚕氨肽酶(BmAPN5)与黑胸败血芽孢杆菌伴胞晶体(PC)毒素相互作用. 生物工程学报, 2017, 33(1): 90-98. |
| [43] | van Frankenhuyzen K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebr Pathol, 2009, 101(1): 1-16. |
| [44] | Crickmore N, Baum J, Bravo A, et al. Bacillus thuringiensis toxin nomenclature. 2016. http://www.btnomenclature.info/. |
| [45] | Jurat-Fuentes JL, Adang MJ. The Heliothis virescens cadherin protein expressed in Drosophila S2 cells functions as a receptor for Bacillus thuringiensis Cry1A but not Cry1Fa toxins. Biochemistry, 2006, 45(32): 9688-9695. DOI:10.1021/bi0606703 |
| [46] | Rajagopal R, Agrawal N, Selvapandiyan A, et al. Recombinantly expressed isoenzymic aminopeptidases from Helicoverpa armigera (American cotton bollworm) midgut display differential interaction with closely related Bacillus thuringiensis insecticidal proteins. Biochem J, 2003, 370(3): 971-978. DOI:10.1042/bj20021741 |
| [47] | Badran AH, Guzov VM, Huai Q, et al. Continuous evolution of Bacillus thuringiensis toxins overcomes insect resistance. Nature, 2016, 533(7601): 58-63. DOI:10.1038/nature17938 |
| [48] | Xiao YT, Dai Q, Hu RQ, et al. A single point mutation resulting in cadherin mislocalization underpins resistance against Bacillus thuringiensis toxin in cotton bollworm. J Biol Chem, 2017, 292(7): 2933-2943. DOI:10.1074/jbc.M116.768671 |
| [49] | Lin P, Cheng TC, Ma SY, et al. Bacillus bombysepticus α-toxin binding to g protein-coupled receptor kinase 2 Regulates cAMP/PKA signaling pathway to induce host death. PLoS Pathog, 2016, 12(3): e1005527. DOI:10.1371/journal.ppat.1005527 |
