
 , 刘丹1, 刘永琦1,2, 薛娜1, 颜春鲁1, 苏韫1
, 刘丹1, 刘永琦1,2, 薛娜1, 颜春鲁1, 苏韫11 甘肃中医药大学 基础医学院,甘肃 兰州 730000;
2 甘肃省高校重大疾病分子医学与中医药防治重点实验室,甘肃 兰州 730000
收稿日期:2017-01-21;接收日期:2017-02-16; 网络出版时间:2017-03-31 基金项目:甘肃省自然科学基金(No. 1212RJ2A081),甘肃省省属普通本科高校基本科研业务费专项(No. 2305136301)资助
摘要:探讨黄芪多糖(APS)对低氧环境中骨髓间充质干细胞(BMSCs)成脂诱导分化的影响。采用四甲基偶氮唑盐(Methyl thiazolyl tetrazolium,MTT)法筛选促进BMSCs增殖的最佳APS浓度,干预不同氧浓度下(3%、6%、10%和20%)成脂诱导培养的BMSCs,通过油红O染色观察细胞内脂滴形成,Real-time PCR和Western blotting检测成脂相关基因过氧化物酶体增殖物激活受体γ2 (PPAR-γ2)和脂蛋白脂肪酶(LPL)的mRNA和蛋白水平。结果表明,与对照组相比,40 μg/mL APS能显著促进不同氧浓度下BMSCs的增殖(P < 0.05);含有40 μg/mL APS的成脂诱导剂能提升低氧环境中BMSCs内脂滴含量及PPAR-γ2和LPL的蛋白和mRNA水平,在氧浓度为10%时其促进作用较显著(P < 0.05),差异有统计学意义。40μg/mL APS具有促进低氧环境中BMSCs增殖和成脂诱导分化的作用,其促分化作用与细胞培养的氧环境相关。
关键词:骨髓间充质干细胞 低氧环境 黄芪多糖 成脂分化
Effects of Astragalus polysaccharides on adipogenic differentiation of bone marrow mesenchymal stem cells in low oxygen environment
Zhiwei Wu1,2

 , Dan Liu1, Yongqi Liu1,2, Na Xue1, Chunlu Yan1, Yun Su1
, Dan Liu1, Yongqi Liu1,2, Na Xue1, Chunlu Yan1, Yun Su1 1 Basic Medical College, Gansu University of Chinese Medicine, Lanzhou 730000, Gansu, China;
2 Provincial-level Key Laboratory for Molecular Medicine of Major Diseases and the Prevention and Treatment with TCM Research in Gansu Colleges and Universities, Lanzhou 730000, Gansu, China
Received: January 21, 2017; Accepted: February 16, 2017; Published: March 31, 2017
Supported by: Natural Science Foundation of Gansu Province (No. 1212RJ2A081), Gansu Provincial General Undergraduate Colleges and Universities Fundamental Research Funds (No. 2305136301)
Corresponding author:Zhiwei Wu. Tel: +86-931-8721601; Fax: +86-931-8765344; E-mail: wzhiwei@aliyun.com
Abstract: To study the effect of Astragalus polysaccharide (APS) on adipogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) cultured in hypoxic environment. The optimal APS concentration, which could promote the proliferation of BMSCs, was screened by methyl thiazolyl tetrazolium method. The concentration was used to intervene in BMSCs-induced by adipogenic differentiation fluid growing in different oxygen concentrations (3%, 6%, 10% and 20%). The formation of lipid droplets in the BMSCs-intervened was observed by oil red O staining under the optical microscope. The mRNA and protein levels of the lipid relating genes peroxisome proliferator activated receptor gamma 2 (PPAR-γ2) and lipoprotein lipase (LPL) were detected by Real-time PCR and Western blotting, respectively. The results showed that, comparing with the control group, 40 μg/mL APS could significantly promote the proliferation of BMSCs under low oxygen concentration. A large amount of lipid droplets existed in BMSCs growing in the adipogenic inducing fluid containing 40 μg/mL APS and the hypoxic environment, and the protein and mRNA levels of PPAR-γ2 and LPL also raised. It was worth noting that the phenomenon was more significant in 10% oxygen concentration, and the difference was statistically significant (P < 0.05). 40 μg/mL APS had effect on promoting the proliferation and adipogenic differentiation of BMSCs cultured in hypoxic environment, and the effect was related to the concentration of oxygen of BMSCs-cultured.
Key words: bone mesenchymal stem cells low oxygen environment Astragalus polysaccharides adipogenic differentiation
来源于中胚层的骨髓间充质干细胞(Bone mesenchymal stem cells,BMSCs)因其具有自我更新和多向分化的潜能,而被作为理想的生物工程种子细胞,广泛应用于组织修复、基因工程和细胞治疗等临床领域[1-3]。然而,大量研究表明,包括BMSCs在内的多能干细胞,极易受到炎性介质[4]、X射线[5]、低氧环境[6-7]等因素的影响,引起细胞内基因转录、翻译及修饰过程的改变,从而导致细胞形态、结构和分化异常,甚至造成BMSCs恶性转变,极大地限制了BMSCs的临床应用和开发前景。
氧作为生物机体新陈代谢的重要营养组分,在促进和维持生物体生长发育以及细胞增殖和分化过程中发挥着重要作用。机体不同组织或体液中氧浓度分布客观地反映了生物体新陈代谢的特殊性,这也为解析组织器官功能和细胞分化与更新提供了理论依据。同时,黄芪多糖(Astragalus polysaccharides)作为黄芪主要有效活性成分之一,具有抗炎、抗肿瘤、免疫调节和促进细胞增殖的功效[8-10]。低氧环境与APS结合起来是否具有促进BMSCs分化的作用?本研究以此为出发点,模拟体内低氧环境,应用APS干预成脂诱导的BMSCs,探析APS对低氧环境中BMSCs成脂分化的作用,这将为解析黄芪多糖的临床疗效和开发应用提供理论依据。
1 材料1.1 BMSCs细胞株Wistar大鼠BMSCs细胞株由本实验室分离鉴定获得。
1.2 主要试剂与仪器DMEM/F12培养基、胎牛血清、0.25%胰蛋白酶和青链霉素(Gibco);成脂诱导培养基(赛业生物科技有限公司);油红O染色试剂盒(杰美基因);RNA提取试剂盒(TaKaRa);PrimeScript RT reagent Kit和SYBR Premix EX Taq (Roche);大鼠PPAR-γ2、LPL单克隆抗体和羊抗鼠IgG-HRP (Abcam);PPARγ2和LPL引物(生工生物工程(上海)股份有限公司);倒置显微镜(OLMYPUS);Real-Time PCR仪(Bio-Rad);CO2细胞培养箱(SANYO)。
2 方法2.1 APS对低氧环境中BMSCs增殖的影响2.1.1 实验分组依据氧浓度及APS浓度,实验共分为24组,其中氧浓度分为3%、6%、10%和20%四个梯度,黄芪多糖浓度分为0、10、20、40、80和100 μg/mL6个梯度,共计4×6组。
2.1.2 细胞培养常规培养第3代(G3) BMSCs至融合达到80%-90%时,将其消化制成单细胞悬液,用完全培养基(10% FBS的高糖DMEM/F12)调整细胞浓度至1×104cells/cm2,接种于96孔板(200 μL/well),细胞终浓度为2×103cells/well;置于常氧环境、37 ℃、5% CO2、相对湿度100%的培养箱中培养24 h (即细胞贴壁)后,弃上清,依次加入含有不同浓度APS的完全培养基(200 μL/well),每浓度3个重复;置于不同氧浓度、37 ℃、5% CO2、相对湿度100%的培养箱中培养。
2.1.3 MTT检测分别取培养1 d、2 d、3 d、4 d、5 d、6 d和7 d的上述BMSCs,弃培养液,用PBS洗涤2遍后,依次加入含0.5%的MTT完全培养基(200 μL/well),于对应氧浓度下继续培养4 h;弃培养液,加入二甲基亚砜(150 μL/well),置摇床低速振荡10 min,应用酶标仪OD490滤光片检测各孔吸光值。
2.2 APS对低氧环境中BMSCs成脂诱导分化的影响2.2.1 实验分组实验共分为空白组、黄芪多糖组、诱导组和诱导剂+黄芪多糖组,同时设立3%、6%、12%和20%四个氧浓度梯度,共计4×4组。
2.2.2 细胞培养常规培养G3代BMSCs至融合达到80%-90%时,用含0.25%胰蛋白酶的D-Hanks液消化,按2×104cells/cm2细胞密度接种于6孔板,每孔加入2 mL完全培养基,置于常氧环境、37 ℃、5% CO2、相对湿度100%的培养箱中培养,至细胞融合达到100%,弃完全培养基。空白对照组加完全培养基、黄芪多糖组加含有40 μg/mL黄芪多糖的完全培养基、诱导组加入诱导培养液A和B (具体操作见Cyagen Biosciences lnc RAWMX 90031说明书)、诱导剂+黄芪多糖组加入含有40 μg/mL黄芪多糖成脂诱导培养液A和B (具体操作同诱导剂组),2 mL/well;置于3%、6%、10%和20%四个氧浓度环境,每隔3 d换液1次,持续培养22 d。
2.2.3 油红O染色分析成脂诱导分化结束后,弃培养液,用PBS洗涤2次,每孔加入2 mL 4%中性甲醛于4 ℃固定30 min;弃甲醛,PBS洗涤2次,每孔加入1 mL油红O染液染色30 min;弃染液,PBS洗涤2次,于相差显微镜下观察脂滴形成。
2.2.4 Real-time PCR分析提取2.2.2培养细胞的总RNA,以表 1序列为引物,采用Real-time PCR检测成脂相关基因PPAR-γ2和LPL的mRNA水平,具体操作见RNA提取试剂盒(TaKaRa)、PrimeScript RT reagent kit和SYBR premix EX Taq (Roche)说明书。
表 1 引物序列Table 1 Primer sequences
| Gene name | Primer sequence (5′-3′) | |
| β-actin | Forward | AGGGAAATCGTGCGTGACAT |
| β-actin | Reverse | GGAAAAGAGCCTCAGGGCAT |
| PPAR-γ2 | Forward | ATAAGGGACGCGAGGAGGTCA |
| PPAR-γ2 | Reverse | AGGGGTGTGGATTTGGTTGTG |
| LPL | Forward | TCAGAAGACATCAGGCAGGAGA |
| LPL | Reverse | AAAGGCCAGGTGTTTCAACCG |
表选项
2.2.5 Western blotting分析提取2.2.2培养细胞的总蛋白,经SDS-PAGE分离后,转印至PVDF膜;将膜置于3%脱脂奶粉中4 ℃过液封闭,弃封闭液;分别加入1:500大鼠PPAR-γ2和LPL单克隆抗体(Abcam),室温孵育4 h,弃一抗,用PBST洗涤3次;加入1:600二抗,室温孵育2 h,弃二抗,应用Western blotting ECL化学发光试剂盒(Bio-Rad)进行染色,具体操作见说明书;将膜置于凝胶成像系统进行分析。
2.3 数据分析应用SPSS 16.0对获得数据进行统计分析,结果用x±s表示,采用多因素方差分析进行各组间及组内均数比较。*P < 0.05为显著,**P < 0.01为极显著。
3 结果与分析3.1 APS对低氧环境中BMSCs增殖的分析由MTT分析显示,在不同氧浓度下,不同黄芪多糖浓度对BMSCs增殖有不同程度的影响。与对照组相比,当氧浓度在3%、6%、10%和20%时,10 μg/mL和20 μg/mL黄芪多糖对BMSCs增殖作用不显著,40 μg/mL黄芪多糖对BMSCs有明显的促增殖作用;当黄芪多糖浓度在80 μg/mL和100 μg/mL时,对BMSCs增殖有一定的抑制作用;当氧浓度在6%、10%和20%时,40 μg/mL黄芪多糖对BMSCs促增殖作用显著(P < 0.05),差异具有统计学意义,见图 1。
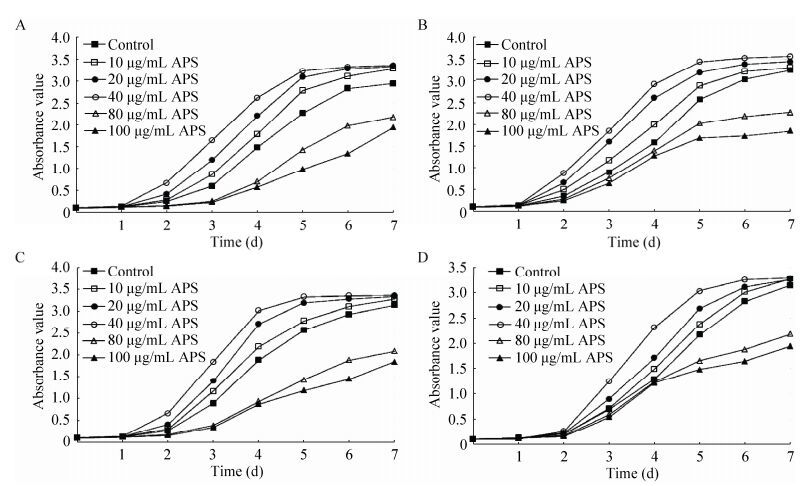 |
| 图 1 黄芪多糖干预的不同氧环境中BMSCs增殖曲线(A-D:3%、6%、10%和20%的氧浓度) Figure 1 Proliferation curve of BMSCs-intervened by Astragalus polysaccharide under hypoxic environment. A-D represent 3%, 6%, 10% and 20% oxygen concentration, respectively. |
| 图选项 |
3.2 油红O染色分析由图可知,在3%、6%、10%和20%四个氧浓度下,空白组和黄芪多糖组细胞内均不存在或有极少量的棕红色脂滴,两组间差异不显著(P > 0.05),无统计学意义;而在诱导组和诱导剂+黄芪多糖组中,细胞内出现了大量的棕红色脂滴,诱导剂+黄芪多糖组高于诱导组(P < 0.05),与空白组和黄芪多糖组相比差异极显著(P < 0.01),具有统计学意义;且在10%氧浓度下,诱导组和诱导剂+黄芪多糖组细胞内脂滴含量最高,见图 2。
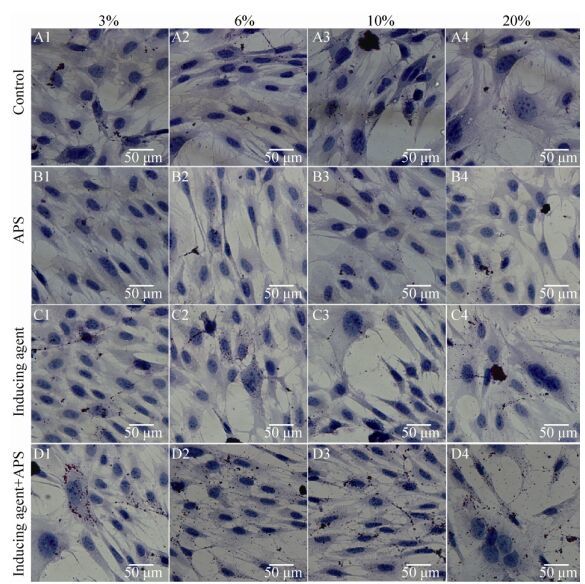 |
| 图 2 成脂诱导BMSCs油红O染色分析(A-D:分别代表空白组、黄芪多糖组、诱导组和诱导剂+黄芪多糖组,1-4分别代表 3%、6%、10%和20%的氧浓度) Figure 2 Analysis of formation of lipid droplets in the BMSCs by oil red O staining. (A) Control group. (B) Astragalus polysaccharide group. (C) Induced group. (D) Inducing agent+Astragalus polysaccharide group. 1-4 represent 3%, 6%, 10% and 20% oxygen concentration, respectively. |
| 图选项 |
3.3 Real-time PCR分析在3%、6%、10%和20%四个氧浓度下,空白组和黄芪多糖组细胞中成脂相关基因PPAR-γ2 (图 3A)和LPL (图 3B)的mRNA水平显著低于诱导剂组和诱导剂+黄芪多糖组(P < 0.01),诱导剂+黄芪多糖组高于诱导剂组(P < 0.05),差异具有统计学意义;空白组和黄芪多糖组间差异不显著(P > 0.05);同时,在诱导剂组和诱导剂+黄芪多糖组中,10%氧浓度下的PPAR-γ2和LPL mRNA水平显著高于其他氧浓度(P < 0.05),见图 3。
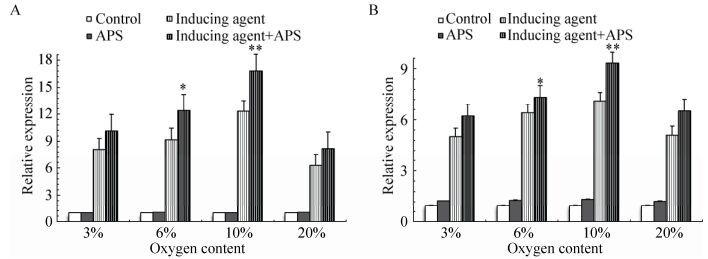 |
| 图 3 PPAR-γ2和LPL mRNA水平的qPCR分析 Figure 3 Analysis of the levels of PPAR-γ2 (A) and LPL (B) mRNA by qPCR. |
| 图选项 |
3.4 Western blotting分析在3%、6%、10%和20%四个氧浓度下,空白组和黄芪多糖组细胞中成脂相关基因PPAR-γ2 (图 4A)和LPL (图 4B)的蛋白水平显著低于诱导剂组和诱导剂+黄芪多糖组(P < 0.01),诱导剂+黄芪多糖组高于诱导剂组(P < 0.05),差异具有统计学意义;空白组和黄芪多糖组间差异不显著(P > 0.05);同时,在诱导剂组和诱导剂+黄芪多糖组中,10%氧浓度下的PPAR-γ2和LPL蛋白水平显著高于其他氧浓度(P < 0.05),见图 4。
 |
| 图 4 成脂相关蛋白PPAR-γ2 (A)和LPL (B)的Western blotting分析 Figure 4 Analysis of the levels of PPAR-γ2 (A) and LPL (B) adipogenic protein by Western blotting. |
| 图选项 |
4 讨论BMSCs是一类具有自我复制和多向分化潜能的多能干细胞,在不同的诱导和培养条件下,可分化为脂肪、骨、软骨、肌肉、肌腱、神经、肝、心肌、内皮等多种组织细胞[11-13],已被用于心脑血管疾病、肝硬化、骨质疏松症、脊髓神经损伤、老年痴呆及红斑狼疮和硬皮病等自身免疫性疾病的治疗,取得了令人鼓舞的临床试验结果[14-16]。目前,部分****应用脂肪细胞共培养技术、MicroRNA调控、激素干预等方式研究BMSCs成脂分化的机制,证实这些因素在抑制或促进BMSCs成脂诱导分化中具有重要的作用[17-19]。因此,依据BMSCs生物学特性,通过药物调节BMSCs多向分化进行临床疾病治疗具有广阔的应用前景。
黄芪多糖(APS)作为黄芪的主要组分在抗氧化、清除自由基、调节细胞基因表达、抗炎、抗肿瘤等方面具有重要的作用。近年来,有大量研究证实黄芪多糖在促进或抑制细胞增殖与分化、调节细胞凋亡、降低细胞毒性等方面具有一定的功效[20]。同时,体内微环境是BMSCs增殖与分化的温床,不同组织或器官中氧浓度分布存在一定的差别,如动脉血氧浓度约为12%,骨髓组织中的氧浓度约为1%-7%,成熟肌肉组织中的氧浓度约为l%-10%21]。氧元素作为体内环境的重要组分之一,通过调节体内基因表达、信号通路等方式在细胞的增殖与分化程中发挥着重要的作用。目前,对于BMSCs增殖与分化的相关研究大多基于常氧环境的细胞培养,并不能真实地反映其在体内的生物学特性。
本研究模拟体内低氧环境,以3%、6%、10%和20%为细胞培养的微环境氧浓度,经MTT法筛选出有效促进BMSCs增殖的最佳APS浓度为40 μg/mL,浓度过高(80-100 μg/mL)抑制BMSCs增殖,浓度过低(40 μg/mL以下)促增殖效果不明显,这一结果与前人研究一致[22]。同时,以40 μg/mL APS干预低氧环境中成脂诱导的BMSCs,通过脂滴形成检测表明,黄芪多糖干预诱导组BMSCs细胞内形成了大量棕红色脂滴,显著高于单独应用诱导剂诱导的BMSCs (P < 0.05),差异具有统计学意义;并且在氧浓度为10%时,其干预诱导作用较为显著。这一结果证实APS不仅具有促进低氧环境中BMSCs成脂诱导分化的功效,而且其促诱导分化作用与BMSCs所处微环境的氧浓度有一定关系。
PPAR-γ是脂肪细胞形成的关键调控因子,该因子具有激活控制BMSCs进入最终分化和获得成熟脂肪细胞功能基因的作用。PPAR-γ的持续表达是维持细胞成脂分化状态的必要条件,具有诱导BMSCs向脂肪细胞分化、促进甘油三酯(TG)合成的作用,一般在成脂向分化中后期高表达[23]。LPL是TG降解的限速酶,对清除体内过多的TG至关重要,在脂代谢、胰岛素抵抗、脂肪细胞分化中有重要作用,成为当今研究肥胖症和相关疾病的热点。PPAR-γ作为LPL表达的影响因素之一,共同调控着BMSCs成脂分化的过程[24]。本研究通过Real-time PCR和Western blotting对低氧环境中APS干预的成脂诱导BMSCs内PPAR-γ和LPL mRNA和蛋白水平分析表明,其表达水平均上调;而且在10%的氧浓度时,APS促PPAR-γ和LPL的mRNA和蛋白水平效果越显著。这一结果提示APS可能通过调控PPAR-γ和LPL的mRNA和蛋白水平来促进低氧环境中BMSCs成脂诱导分化的过程,而且其促诱导分化作用与BMSCs所处微环境的氧浓度有一定关系,其具体机制有待进一步研究。
参考文献
| [1] | Gu Z, Akiyama K, Ma X, et al. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice.Lupus, 2010, 19(13): 1502–1514.DOI: 10.1177/0961203310373782 |
| [2] | Gu ZF, Meng Y, Tao T, et al. Endoplasmic reticulum stress participates in the progress of senescence of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus.Cell Tissue Res, 2015, 361(2): 497–508.DOI: 10.1007/s00441-015-2131-x |
| [3] | Jang E, Jeong M, Kim S, et al. Infusion of human bone marrow-derived mesenchymal stem cells alleviates autoimmune nephritis in a lupus model by suppressing follicular helper T-cell development.Cell Transplant, 2016, 25(1): 1–15.DOI: 10.3727/096368915X688173 |
| [4] | Abdallah BM, Boissy P, Tan QH, et al. dlk1/FA1 regulates the function of human bone marrow mesenchymal stem cells by modulating gene expression of pro-inflammatory cytokines and immune response-related factors.J Biol Chem, 2007, 282(10): 7339–7351.DOI: 10.1074/jbc.M607530200 |
| [5] | Liu HP. Effect of low dose X-ray irradiation on osteogenic differentiation of rat bone marrow mesenchymal stem cells.China Prac Med, 2012, 7(17): 246–247.(in Chinese). 刘洪鹏. 低剂量X线照射对诱导大鼠骨髓间充质干细胞成骨分化的影响.中国实用医药, 2012, 7(17): 246-247.DOI:10.3969/j.issn.1673-7555.2012.17.195 |
| [6] | Naqvi SM, Buckley CT. Extracellular matrix production by nucleus pulposus and bone marrow stem cells in response to altered oxygen and glucose microenvironments.J Anat, 2015, 227(6): 757–766.DOI: 10.1111/joa.12305 |
| [7] | Lu HT, Yuan F, Zhang JW, et al. Osteogenic capability of co-cultured rabbit periosteal cells and nucleus puplousus cells in hypoxic conditions.Orthop J China, 2016, 24(9): 839–844.(in Chinese). 陆海涛, 袁峰, 张峻玮, 等. 低氧环境下共培养的骨膜细胞和髓核细胞骨向分化能力的研究.中国矫形外科杂志, 2016, 24(9): 839-844. |
| [8] | Zhang CL, Ren HJ, Liu MM, et al. Modulation of intestinal epithelial cell proliferation, migration, and differentiation in vitro by astragalus polysaccharides.PLoS ONE, 2014, 9(8): e106674.DOI: 10.1371/journal.pone.0106674 |
| [9] | Wang XM, Jia TY, Guan B, et al. Effects of astragalus polysaccharide on CD4+ CD25+ treg cells and Th17 cells in immunesuppressed mice.Nat Prod Res Dev, 2015, 27(1): 153–157.(in Chinese). 王雪梅, 贾天玉, 管彬, 等. 黄芪多糖对免疫抑制模型小鼠Treg细胞及Th17细胞亚群的影响.天然产物研究与开发, 2015, 27(1): 153-157. |
| [10] | Luo ZF, Su XC, Kong JX, et al. The influence of astragalus polysaccharide on Qi-deficiency and blood-stasis in rats induced by cyclophosphamide.J Mod Oncol, 2016, 24(1): 30–32.(in Chinese). 罗泽飞, 苏旭春, 孔嘉欣, 等. 黄芪多糖对环磷酰胺导致大鼠气虚血瘀证的影响.现代肿瘤医学, 2016, 24(1): 30-32. |
| [11] | Jiang YH, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow.Nature, 2002, 418(6893): 41–49.DOI: 10.1038/nature00870 |
| [12] | Wang C, Meng HY, Wang X, et al. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis.Med Sci Monit, 2016, 22: 226–233.DOI: 10.12659/MSM.897044 |
| [13] | Lü S, Wu L, Cheng P, et al. Correlation of obesity and osteoporosis: effect of free fatty acids on bone marrow-derived mesenchymal stem cell differentiation.Exp Ther Med, 2010, 1(4): 603–610.DOI: 10.3892/etm_00000095 |
| [14] | Sun LY, Akiyama K, Zhang HY, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans.Stem Cells, 2009, 27(6): 1421–1432.DOI: 10.1002/stem.v27:6 |
| [15] | Fan YX, Zhang YL, Wang Q, et al. Overexpression of connexin 43 in bone marrow mesenchymal stem cells in Xiao Meishan swines.Chin J Biotech, 2015, 31(3): 351–360.(in Chinese). 樊懿萱, 张艳丽, 王强, 等. 小梅山猪骨髓间充质干细胞Cx43基因修饰.生物工程学报, 2015, 31(3): 351-360. |
| [16] | González MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells.Arthritis Rheum, 2009, 60(4): 1006–1019.DOI: 10.1002/art.v60:4 |
| [17] | Gao F, Lin M, Ge ZM, et al. Effect of parathyroid hormone on LPL and ALP during adipogenic differentiation by rat bone marrow mesenchymal stem cells.Chin J Osteoporos, 2015, 21(12): 1460–1464.(in Chinese). 高飞, 林梅, 葛志敏, 等. 甲状旁腺激素对大鼠骨髓间充质干细胞成脂分化中LPL和ALP的影响.中国骨质疏松杂志, 2015, 21(12): 1460-1464.DOI:10.3969/j.issn.1006-7108.2015.12.008 |
| [18] | Bi Y, Gong M, He Y, et al. Recombinant adenovirus expressing siRNA is generated to inhibit the expression of RARβ in rat mesenchymal stem cells treated by all-trans retinoic acid.Chin J Biotech, 2012, 28(5): 632–642.(in Chinese). 毕杨, 龚敏, 何昀, 等. 腺病毒介导siRNA抑制全反式维甲酸诱导的骨髓间充质干细胞RARβ表达.生物工程学报, 2012, 28(5): 632-642. |
| [19] | Tang YF, Zhang Y, Li XY, et al. Expression of miR-31, miR-125b-5p, and miR-326 in the adipogenic differentiation process of adipose-derived stem cells.OMICS, 2009, 13(4): 331–336.DOI: 10.1089/omi.2009.0017 |
| [20] | Fang JK, Zhou YP, Li ML. Research progress on effects of traditional Chinese medicines on proliferation, apoptosis and differentiation of bone marrow mesenchymal stem cells.China J Chin Mater Med, 2014, 39(15): 2834–2837.(in Chinese). 方健康, 周轶平, 李玛琳. 中药对骨髓间充质干细胞增殖、凋亡及分化的影响研究进展.中国中药杂志, 2014, 39(15): 2834-2837. |
| [21] | Chakravarthy MV, Spangenburg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles.Cell Mol Life Sci, 2001, 58(8): 1150–1158.DOI: 10.1007/PL00000929 |
| [22] | Hou Q, Zhang QJ, Liu YQ, et al. Effect of Astragalus injection on proliferation of human bone marrow mesenchymal stem cells cultured in hypoxic environment and vitro.Chin Trad Patent Med, 2015, 37(4): 873–875.(in Chinese). 侯茜, 张秋菊, 刘永琦, 等. 低氧环境下黄芪注射液对人骨髓间充质干细胞体外增殖的影响.中成药, 2015, 37(4): 873-875. |
| [23] | Hong JH, Hwang ES, McManus MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation.Science, 2005, 309(5737): 1074–1078.DOI: 10.1126/science.1110955 |
| [24] | Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity.Am J Physiol Endocrinol Metab, 2009, 297(2): E271–E288.DOI: 10.1152/ajpendo.90920.2008 |
