
 , 罗修鑫, 鞠环宇, 安东, 刘莹, 王君伟
, 罗修鑫, 鞠环宇, 安东, 刘莹, 王君伟

东北农业大学 动物医学学院,黑龙江 哈尔滨 150030
网络出版时间:2016-07-14
摘要: 免疫球蛋白是机体固有免疫系统的组成部分,是机体防御的第一道防线。本研究对抗鹅免疫球蛋白轻链单克隆抗体进行了特征分析并将其应用到不同免疫试验中用以检测鹅免疫球蛋白。用此单克隆抗体制备的免疫亲和层析柱用以分离血清中的鹅免疫球蛋白;偶联辣根过氧化物酶(Horseradish peroxidase,HRP)后的单克隆抗体用作第二抗体来检测鹅特异性抗体。此外,该单克隆抗体可以识别和定位外周血淋巴细胞中的SIg+淋巴细胞。研究表明,该单克隆抗体可在多种条件下检测或分离鹅免疫球蛋白并作为研究鹅体液免疫的有力工具。
关键词: 单克隆抗体 鹅免疫球蛋白 轻链 免疫试验
Characterization and application of a monoclonal antibody against light chain of goose immunoglobulin
Yongli Guo, Mingchun Gao

 , Xiuxin Luo, Huanyu Ju, Dong An, Ying Liu, Junwei Wang
, Xiuxin Luo, Huanyu Ju, Dong An, Ying Liu, Junwei Wang

College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, Heilongjiang, China
Received: April 12, 2016; Accepted: June 20, 2016
Supported by:Northeast Agricultural University Innovation Foundation for Postgraduate (No. yjscx14013)
Corresponding authors:Mingchun Gao. Tel/Fax: +86-451-55191672; E-mail: gaomingchun@neau.edu.cn
Junwei Wang. jwwang@neau.edu.cn
Abstract: Immunoglobulin (Ig) is considered a part of the innate immune system and cooperates with the complementary system as the first line of defense. In this study, a monoclonal antibody (MAb) direct against the light chain of goose Ig (GoIgCL) was generated, characterized and identified in various immunoassays to detect goose Ig. An immunoaffinity chromatography column prepared with this MAb was used to separate the goose Ig from sera. After being conjugated with horseradish peroxidase (HRP), this MAb was used as the secondary antibody to evaluate the goose-specific antibody. In addition, this MAb distinguished and localized the SIg+ lymphocytes from peripheral blood lymphocytes. MAb against GoIgCL may be good candidate to detect or purify goose Ig under various conditions and as a powerful tool for humoral immunity research on goose.
Key words: monoclonal antibody goose immunoglobulin light chain immunoassay
IntroductionImmunoglobulin (Ig), an important effector molecule of the humoral immunity, is considered a part of the innate immune system and has been suggested to cooperate with the complementary system in mammals as a first line of defense[1], which can be produced either in secreted form or as a surface Ig (SIg) receptor form. Birds have three classes of antibodies, namely, IgM, IgY, and IgA, present in serum and secretions with a distinct tissue distribution, and different studies have shown only one type of light chain called Igλ existing in bird systems, and Ig in goose is homologous to those in other birds[1]. Therefore, to some extent, the immunological properties of light chain can represent that of intact Ig in geese.
Poultry are the natural reservoir of human pathogens, which are a threat on the economy and human health. This makes the detections and surveys of poultry infectious diseases essential to human health[2]. The tortoise SIgM+ and SIgY+ lymphocytes in blood and spleen were identified with the antisera to tortoise IgM and IgY produced in mice[3]. Monoclonal antibodies (MAbs) to mucus Ig of flounder were used to analyze the relationships among mucus Ig, serum Ig, and SIg+ lymphocytes in different tissues of flounder[4]. The chicken Ig isotypes G, M, and A were quantitatively and qualitatively evaluated with the MAbs specific for chicken IgG, IgM, or IgA[5]. The Newcastle disease virus immunoassays for waterfowl was established with a MAb specific for the duck Ig light chain[6], while lacking availability of the appropriate tools make limited research on goose. Affinity chromatography is a powerful method to purify recombinant proteins from many genera. Immunoaffinity chromatography has also been utilized in the purification of recombinant human TPB protein and GFP fusion proteins[7-8], In our previous research, we prepared the MAb against the constant region of goose immunoglobulin light chain (GoIgCL), which can react with GoIgCL from sera and bile[9]. In this study, we further studied the characterization and the application of this MAb in various immunoassays, such as antibody surveillance, immunoaffinity purification, quantitative identification and location analysis of SIg on lymphocytes. The results suggest that this MAb can be used as a specific reagent for detection of goose disease-specific antibodies and as a powerful tool for goose basic immunology research.
1 Materials and methods1.1 Cells, sera samples, and animalsBHK21 cells were used for transient expression. Hybridoma cells secreting antibody against GoIgCL were described previously[9]. Serum samples collected from geese immunized with the goose parvovirus (GPV) recombinant VP2-derived virus-like particles (rVP2-VLPs), recombinant VP3-derived virus-like particles (rVP3-VLPs), live attenuated vaccine and inactivated vaccine were prepared described previously[10]. BALB/c mice (5-6 weeks old, female) were purchased from the Veterinary Institute in Harbin. The 18-month-old white geese were purchased from Datong Farm near Harbin. All animals and animal subjects used in this research have been approved by the Scientific Ethical Committee of the Northeast Agricultural University.
1.2 Ascetic fluid preparation and purificationAdult mice were intraperitoneally injected with 0.5 mL of paraffin. After 7 days, 106 hybridoma cells secreting the anti-GoIgCL MAb in 0.5 mL of PBS were injected into intraperitoneal cavity of each mouse. After another 7-14 days, the ascetic fluid was drawn using an 18-gauge needle attached to a 5 mL syringe[11]. The collected fluid was purified with Protein G affinity column (GenScript, Nanjing, China) and analyzed by SDS-PAGE.
1.3 Affinity measurementThe affinity was calculated using the method of Beaty et al.[12]. Different concentrations of goose Ig were coated against different MAb concentrations. By using the formulas presented in this method (Kaff=1/2(2[Ab’]t -[Ab]t), the affinity constant was determined.
1.4 Preparation and usage of immunoaffinity chromatography columnPurified MAb (10 mg) was coupled with NHS-activated Sepharose 4B (WSAC, Beijing, China) according to the instructions. Filtered goose serum (1 mL) diluted with different binding buffer was then loaded onto the column. Afterward, the column was washed with 10 mL of binding buffer and eluted with 10 mL of freshly prepared elution buffer. The eluted fractions were collected in a tube containing 1 mol/L Tris-base buffer to immediately neutralize the pH to 7.4 before use. SDS-PAGE was used to analyze the purification effect with the prepared column with MAb.
1.5 Antibody detection with the conjugated MAbThe MAb purified from ascetic fluid was conjugated with HRP by CoWin Biotech (Beijing, China) (Contract No: CWBJ-D-20101018A). The working concentration of MAb conjugated with HRP was tested through direct ELISA with recombinant GoIgCL protein rGoCL[13] coated on the plate. Serum samples (five geese per group, four groups, and 0-8th weeks) collected from geese immunized with rVP2-VLPs, rVP3-VLPs, live attenuated vaccine and inactivated vaccine were separately tested by indirect ELISA with MAb conjugated with HRP as the second antibody. The positive serum and negative serum was collected and preserved in our laboratory, which was tested by agar gel precipitation[14]. The recombinant VP2 protein expressed in insect cells[10] was used as the coating antigen with 0.05 mol/L carbonate buffer (pH 9.6) by 4 °C incubation overnight. The plates were then washed three times with PBST (pH 7.4) and then blocked with 5% skimmed milk (Sigma, NY, USA) in PBST for 2 h at 37 °C. After three times washes with PBST, 100 μL of serum (the serum samples after the VLPs and vaccine immunizations, the positive and negative serum) diluted 1?100 in PBST were incubated for 1 h at 37°C. After washing with PBST, 100 μL of MAb conjugated with HRP diluted at 1?4 000 in PBST was added. After 1 h incubation at 37 °C and subsequent washing, the color was developed using the chromogen/substrate mixture TMB/H2O2. The reaction was stopped after 15 min by adding 1 mol/L H2SO4 and the OD450 of each well was read[15]. The controls (in duplicate) included a positive control, a negative control and a blank control.
This MAb was conjugated with FITC according the standard procedure[16]. The recombinant vector pcDNA3.1-GoIgCL contained the GoIgCL sequence amplified with primers CL-F and CL-R, then the plasmid pcDNA3.1 (+) and pcDNA3.1-GoIgCL were transfected into the BHK-21 cells separately with the transfection reagent LipofectamineTM 2000 (Invitrogen, CA, USA) according to the protocol. After incubating for 36 h at 37 °C, the washed cells were fixed with freshly prepared 4% paraformaldehyde (PA) for 15 min at room temperature. After washing three times with PBS, the cells were incubated at 37 °C for 2 h with MAb conjugated with FITC diluted at 1:1 000 in PBS. After washing with PBS four times, the cells were analyzed by fluorescence microscopy.
Table 1 Primers used in this study
| Primer name | Primer sequence (5'-3') |
| CL-F | AGCAAGCTTATGTCTCCCAGCATCTACCTCTTCCCG |
| CL-R | CAGGATCCCTAGTTCAGGGTCTTCTCGGTGACAG |
| The restriction enzyme sites (Hind Ⅲ and Xho Ⅰ) that were introduced in each primer are underlined. | |
表选项
1.6 Identification and location analysis of SIg on lymphocytesThe lymphocytes were isolated from the goose peripheral blood using a lymphocyte separation medium (TBD, Tianjin, China) according to the manufacturer’s instruction.
Flow cytometry was used to analyze the isolated lymphocytes using MAb according to the reported method[17] with some modifications. MAb diluted with 1% PBA (0.01 mol/L PBS, pH 7.4; 1% BSA) at a concentration of 0.2 mg/mL in a 200 μL volume was then mixed with the isolated lymphocytes at 4 °C for 2 h. The cells were subsequently washed with PBS three times and incubated with FITC-conjugated goat anti-mouse (ZSGB, Beijing, China) at 37 °C for 30 min in the dark. After washing, the cells were re-suspended and analyzed by flow cytometry. Cells incubated with the secondary antibody were used as the negative control. At the same time, MAb conjugated with FITC was used as the antibody to incubate with the isolated lymphocytes at 37 °C for 30 min in the dark.
About 1×106 isolated lymphocytes were fixed in 4% PA and permeabilized by 1% Triton X-100. After washing, the MAb 1B11 diluted with 2% PBA (0.01 mol/L PBS, pH 7.4; 2% BSA) and with a concentration of 0.2 mg/mL (400 μL volume) was added. The cells were then washed and incubated with FITC-conjugated goat anti-mouse at 37 °C for 1 h in the dark. Cells incubated with the secondary antibody were used as the negative control. The cells were subsequently stained with DAPI (5 μg/mL) at 37 °C for 20 min and analyzed by confocal microscopy. For double labeling, cells were successively incubated with MAb, polyclonal antibody (PAb) against goose IgY-Fc fragment[18], and then with a mixture of the TRITC-conjugated goat anti-rabbit and FITC-conjugated goat anti-mouse antibodies (ZSGB, Beijing, China). After each step, cells were washed thrice in PBST.
2 Results and discussionAscetic fluid was prepared and purified with Protein G affinity column, SDS-PAGE revealed two specific bands at the location of the heavy and light chains of mice antibody (Fig. 1A). Then affinity constant (Kaff) of 5.12×109 M-1 was calculated, which is suitable for the target combination and meets the standard for the preferred target ligand with an affinity within 106 to 1010 M-1[19].
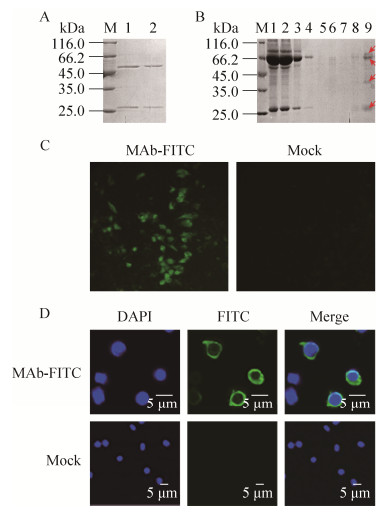 |
| Figure 1 Identification of the anti-GoIgCL MAb. (A) Purification of MAb. Lane M: unstained protein molecular weight marker. Lane 1-2: MAb purified from ascites fluid. (B) Purification of goose Ig from serum through immunoaffinity chromatography column prepared with MAb. Lane M: unstained protein molecular weight marker. Lane 1: sample before purification; Lane 2: unbound protein; Lanes 3-7: protein complex washed with washing buffer; Lanes 8-9: protein eluted with elution buffer. Protein bands in lane 9 at the locations of 86, 67, 42, and 25 kDa are denoted by arrows. (C) Immunofluorescence analysis of the transient transfected cells with MAb conjugated with FITC. (D) Laser scanning confocal microscopy analysis of peripheral blood lymphocytes with MAb conjugated with FITC. SIg+ was stained with FITC (green), and the nucleus was stained with DAPI stain (blue). |
| 图选项 |
Antibodies are usually isolated from plasma, serum, ascites fluid, cell culture medium, egg yolk, plant extracts or bacterial and yeast cultures. All of these sources contain different proteins other than antibodies. Hence, efficient purification of antibodies becomes imperative[20]. MAbs are usually used as important tools in immunoaffinity chromatography[21]. Anti-GoIgL MAb was used to prepare an immunoaffinity chromatography column in order to isolate GoIgs from sera. SDS-PAGE analysis suggested there was more than 50% of anti-GoIgL MAb coupled with the NHS-activated Sepharose 4B (Fig. 1B). With this column prepared, the GoIgs isolated from goose serum are evidently composed of four main bands (Fig. 1C), which may result from the different isotypes of goose Ig, including the light chain and the heavy chain of IgY, the truncated form of IgY and IgM.
The conjugation of enzymes to antibody, particularly MAb, has allowed the universal application of immunological techniques for histochemistry, cytochemistry, and quantitative assays (e.g., ELISA and cytogenetics)[22]. After conjugated with HRP by CoWin Biotech, the concentration of MAb conjugated with HRP was 3 mg/mL, and that of HRP/IgG was 2.0, the working concentration tested through ELISA was 1?4 000. The goose serum samples derived from geese immunized with GPV rVP2-VLPs, rVP3-VLPs, live attenuated vaccine, and inactivated vaccine were used as the primary antibody, and MAb conjugated with HRP was used as the second antibody. The examined antibody levels were gradually increased in the rVP2-VLP and rVP3-VLP groups till at the sixth week, while the serum antibody peaked at the seventh week in the live attenuated vaccine and inactivated vaccine groups (Fig. 2A), which were consistent with the results of the competitive ELISA analysis established using MAb against GPV[14], this result also suggested that this MAb may be used as a universal antibody in detecting or diagnosing the various antibodies produced by the immunized or infected geese body.
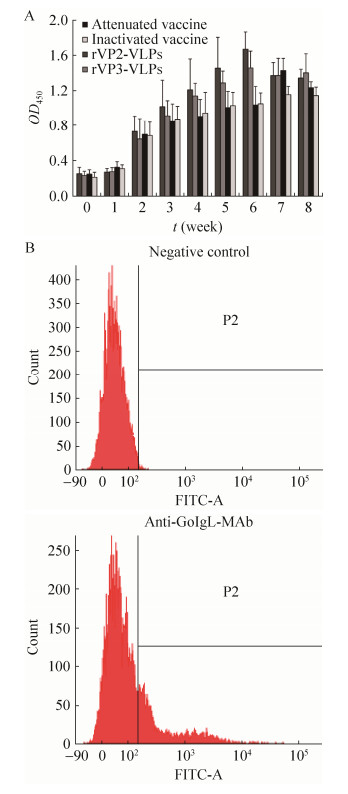 |
| Figure 2 Application of anti-GoIgCL MAb in detecting specific antibody and SIg+ lymphocytes. (A) Evaluation of the goose specific antibodies with MAb. The goose antibodies were detected with MAb conjugated with HRP as the secondary antibody. (B) Flow cytometry analysis of peripheral blood lymphocytes with MAb. The samples free of anti-GoIgL MAb were used as the negative control. |
| 图选项 |
After conjugated with FITC, the concentration of MAb 1B11 conjugated with FITC was 2 mg/mL, and that of the F/P was 7.8, the working concentration tested through immunofluorescence assay was 1:1 000, then the immunofluorescence analysis was performed. The recombinant vector pcDNA3.1-GoIgCL was constructed and transfected into BHK-21 cells, the results showed that this conjugated MAb could bind to BHK-21 cells transfected with pcDNA3.1-GoIgCL expression plasmid, whereas no fluorescence was observed in the negative control (Fig. 1C). At the same time, the reactivity of this conjugated MAb with the isolated lymphocytes expressing SIg was visualized through confocal microscopy (SIg+, green; nucleus, blue) (Fig. 1D). These results suggested that MAb conjugated with FITC can not only bind the GoIgL expressed in BHK-21 cells, but also detect the native Ig on the surfaces of lymphocytes from peripheral blood.
Flow cytometry was conducted to analyze the reactivity of MAb with lymphocytes. The results show that this MAb can recognize the partial of peripheral blood lymphocytes with specific indicator, and the lymphocytes reacted with this MAb took up about 20% of the total lymphocytes isolated from the peripheral blood (Fig. 2B), and the amount of SIg+ lymphocytes that MAb specifically bound to is corresponding to the proportion of B cells in total lymphocytes isolated from the peripheral blood. In addition, there is a higher background (data not present) in the flow cytometry analysis of peripheral blood lymphocytes with this conjugated MAb, which may be due to fractions that contain FITC conjugated to more than one molecule of antibody. To reduce background problems, a trial experiment using different concentrations of MAb 1B11 conjugated with FITC may be better.
Laser scanning confocal microscopy (LSCM) was performed to analyze sIg expression on the surface of lymphocytes with this MAb. The isolated lymphocytes were fixed, permeabilized, incubated with MAb, and then stained with DAPI and anti-rabbit-IgG-FITC antibody. For double labeling, cells were successively incubated with anti-GoIgL MAb, polyclonal antibody (PAb) against goose IgY-Fc fragment, and then with a mixture of the FITC-conjugated goat anti-mouse and anti-rabbit-IgG-TRITC antibody. LSCM analysis indicated that the SIg+, SIgY+ lymphocytes (SIg+, green and SIgY+, red) and a merged image with the stained nucleus (DAPI stain; blue) can be visualized. The green fluorescent labeling for SIg was located on the plasma membrane of lymphocytes (Fig. 3A), the SIg and SIgY were co-localized on the plasmamembrane of lymphocytes with this MAb and PAb against the goose IgY-Fc fragment (Fig. 3B). The SIgY+ lymphocytes also expressed SIg[23], which corresponds to IgY consisting of λ and υ chains, the λ chain is the basic unit of all subtypes of goose Ig. These findings demonstrate that all isotypes of Ig can be expressed as SIg existing on the membrane surface, which is a feature of B cells in most species[24].
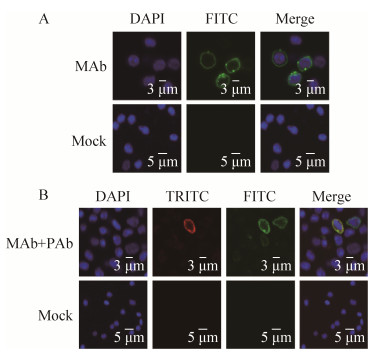 |
| Figure 3 Laser scanning confocal microscopy analysis of peripheral blood lymphocytes with MAb. (A) Detection of SIg+ lymphocytes with MAb. (B) Co-localization analysis of SIg+ and SIgY+ expression on lymphocytes with MAb and PAb against the goose IgY-Fc fragment. SIg+ lymphocytes were detected using FITC (green) and SIgY+ lymphocytes were detected using TRITC (red). The nucleus was stained with DAPI stain (blue). The merged image shows the large nucleus of lymphocytes and the SIg+ and SIgY+ staining were located in the plasma membrane. |
| 图选项 |
As avian species are widely consumed worldwide and represent a source of zoonotic influenza, investigating the composition of their humoral immunity for the development of effective research tools is important. Studies on these research tools for avian humoral immunity are largely restricted to chicken[25-26], which seriously affects the study of avian immune response. The production of MAb against the constant region of goose Ig light chain was reported in our previous study, it can bind all isotypes of goose Ig[9]. In this study, we further characterized this MAb and use this MAb in detecting the goose disease-specific antibodies and as a powerful tool for goose basic immunology research. Moreover, the basic tools and methods for investigating goose immune responses are provided.
参考文献
| [1] | Thornton BP, V?tvicka V, Ross GD. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes.J Immunol,1994, 152(4): 1727–1737. |
| [2] | Reed KD, Meece JK, Henkel JS, et al. Birds, migration and emerging zoonoses: west nile virus, lyme disease, influenza A and enteropathogens.Clin Med Res,2003, 1(1): 5–12.DOI: 10.3121/cmr.1.1.5 |
| [3] | Andreas EM, Ambrosius H. Surface immunoglobulin on lymphocytes of the tortoise Agrionemys horsfieldii.Dev Comp Immunol,1989, 13(2): 167–175.DOI: 10.1016/0145-305X(89)90031-1 |
| [4] | Sheng XZ, Xu GJ, Tang XQ, et al. Monoclonal antibodies recognizing mucus immunoglobulin and surface immunoglobulin-positive cells of flounder (Paralichthys olivaceus).Vet Immunol Immunopathol,2012, 145(1/2): 143–150. |
| [5] | Erhard MH, Von Quistorp I, Schranner I, et al. Development of specific enzyme-linked immunosorbent antibody assay systems for the detection of chicken immunoglobulins G, M, and A using monoclonal antibodies.Poult Sci,1992, 71(2): 302–310.DOI: 10.3382/ps.0710302 |
| [6] | Kothlow S, H?uslaigner R, Kaspers B, et al. Evaluation of Newcastle disease virus immunoassays for waterfowl using a monoclonal antibody specific for the duck immunoglobulin light chain.Avian Pathol,2008, 37(3): 323–328.DOI: 10.1080/03079450802050671 |
| [7] | Zhuang R, Zhang Y, Zhang R, et al. Purification of GFP fusion proteins with high purity and yield by monoclonal antibody-coupled affinity column chromatography.Protein Expr Purif,2008, 59(1): 138–143.DOI: 10.1016/j.pep.2008.01.020 |
| [8] | Thompson NE, Foley KM, Burgess RR. Antigen-binding properties of monoclonal antibodies reactive with human TATA-binding protein and use in immunoaffinity chromatography.Protein Expr Purif,2004, 36(2): 186–197.DOI: 10.1016/j.pep.2004.02.020 |
| [9] | Guo YL, Gao MC, Ma B, et al. A novel monoclonal antibody against the constant region of goose immunoglobulin light chain.Monoclon Antib Immunodiagn Immunother,2014, 33(2): 121–125.DOI: 10.1089/mab.2013.0071 |
| [10] | Ju HY, Wei N, Wang Q, et al. Goose parvovirus structural proteins expressed by recombinant baculoviruses self-assemble into virus-like particles with strong immunogenicity in goose.Biochem Biophys Res Commun,2011, 409(1): 131–136.DOI: 10.1016/j.bbrc.2011.04.129 |
| [11] | Coligan J, Bierer B, Margulies D, et al. edited. Cao XT et al. translated. Short Protocols in Immunology. Beijing: Science Press, 2009: 28-29 科利根, 比勒, 马古利斯(美)等编著.曹雪涛, 等译, 精编免疫学实验指南.北京:科学出版社, 2009: 28-29. |
| [12] | Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay.J Immunol Methods,1987, 100(1/2): 173–179. |
| [13] | Wang JW, Guo YL, Gao MC. Preparation of polyclonal antibody specific for light chain of goose immunoglobulin using recombinant protein.J Northeast Agric Univ,2012, 43(9): 60–63.(in Chinese). 王君伟, 郭永丽, 高明春. 外源重组蛋白制备鹅免疫球蛋白轻链特异性多克隆抗体.东北农业大学学报, 2012, 43(9): 60-63. |
| [14] | Wang Q, Ju HY, Li YW, et al. Development and evaluation of a competitive ELISA using a monoclonal antibody for antibody detection after goose parvovirus virus-like particles (VLPs) and vaccine immunization in goose sera.J Virol Methods,2014, 209: 69–75.DOI: 10.1016/j.jviromet.2014.08.021 |
| [15] | Gao MC, Zhang RX, Li M, et al. An ELISA based on the repeated foot-and-mouth disease virus 3B epitope peptide can distinguish infected and vaccinated cattle.Appl Microbiol Biotechnol,2012, 93(3): 1271–1279.DOI: 10.1007/s00253-011-3815-0 |
| [16] | Haugland RP. Coupling of monoclonal antibodies with fluorophores//Davis WC, Ed. Monoclonal Antibody Protocols. Totowa, N.J.: Humana Press, 1995: 205-221. |
| [17] | Zhang XL, Shao JW, Cao CQ, et al. Development and characterization of monoclonal antibodies against goose CD3ε extracellular domain and their application in detection of CD3+ T lymphocytes.Vet Immunol Immunopathol,2015, 168(3/4): 135–139. |
| [18] | Zhao PP, Guo YL, Ma B, et al. Generation and characterization of polyclonal antibody against part of immunoglobulin constant heavy υ chain of goose.Monoclon Antib Immunodiagn Immunother,2014, 33(4): 287–290.DOI: 10.1089/mab.2013.0092 |
| [19] | Brassfield AL. Antigen purification by monoclonal antibody immunoaffinity chromatography//Davis WC, Ed. Monoclonal Antibody Protocols. Totowa, N.J.: Humana Press, 1995: 195-203. |
| [20] | Ayyar BV, Arora S, Murphy C, et al. Affinity chromatography as a tool for antibody purification.Methods,2012, 56(2): 116–129.DOI: 10.1016/j.ymeth.2011.10.007 |
| [21] | Subramanian A. Immunoaffinity chromatography.Mol Biotechnol,2002, 20(1): 41–47.DOI: 10.1385/MB:20:1 |
| [22] | Haugland RP. Coupling of monoclonal antibodies with enzymes//Davis WC, Ed. Monoclonal Antibody Protocols. Totowa, N.J.: Humana Press, 1995: 235-243. |
| [23] | Guo YL, Gao MC, Zhang HL, et al. Molecular characterization and B cell membrane expression analysis of Fc fragment gene of goose IgY.Res Vet Sci,2014, 97(2): 288–291.DOI: 10.1016/j.rvsc.2014.07.005 |
| [24] | Bird P, Jones P, Allen D, et al. Analysis of the expression and secretion of isotypes of sheep B cell immunoglobulins with a panel of isotype-specific monoclonal antibodies.Res Vet Sci,1995, 59(3): 189–194.DOI: 10.1016/0034-5288(95)90000-4 |
| [25] | Palaniyappan A, Das D, Kammila S, et al. Diagnostics of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid antigen using chicken immunoglobulin Y.Poult Sci,2012, 91(3): 636–642.DOI: 10.3382/ps.2011-01916 |
| [26] | Van Nerom A, Ducatelle R, Haesebrouck F, et al. Monoclonal and polyclonal antibodies to chicken immunoglobulin isotypes specifically detect turkey immunoglobulin isotypes.Vet Immunol Immunopathol,1997, 57(3/4): 305–314. |
