
 , 余小芳1,2, 熊小英1,2, 刘君政1,2, 张华1
, 余小芳1,2, 熊小英1,2, 刘君政1,2, 张华11. 江西师范大学鄱阳湖湿地与流域研究教育部重点实验室, 南昌 330022;
2. 江西师范大学地理与环境学院, 南昌 330022
收稿日期: 2019-04-19; 修回日期: 2019-05-27; 录用日期: 2019-05-27
基金项目: 国家自然科学基金(No.41661017);鄱阳湖湿地与流域研究教育部重点实验室(江西师范大学)开放基金资助项目(No.PK2018001)
作者简介: 王鹏(1982—), 男, 副教授, 博士, 主要从事流域水环境和湖泊生态环境的研究.E-mail: wangpengjlu@jxnu.edu.cn
通讯作者(责任作者): 王鹏
摘要: 本研究采集鄱阳湖湿地3种典型植被(虉草、苔草、芦苇)土壤,在室内分别设置30% WHC(最大持水量)、50% WHC和80% WHC 3种水分条件培养1个月,分别模拟重度干旱、轻度干旱和适宜水分环境,然后添加水分到200% WHC模拟干湿转化过程;基于15N同位素稀释法计算干化-干湿转化过程中湿地土壤的总氨化速率和总硝化速率.土壤干化过程中,芦苇带土壤总氨化速率最高,虉草带土壤总硝化速率最高;土壤总氨化速率和总硝化速率都随干旱程度增强而降低,轻度干旱条件下总硝化速率的降低比总氨化速率更明显;除水分条件外,总氨化速率主要受土壤碳含量影响,总硝化速率主要受pH值影响.土壤湿化过程中,苔草带和芦苇带土壤氮总氨化速率在1 d内变化较小,1~5 d不断下降;虉草带重度干旱土壤氮总氨化速率在湿化后呈上升趋势,轻度干旱土壤只在湿化后1 d内明显增大;3种植被土壤总硝化速率都在1 d内明显下降,此后维持较低水平.干化过程中,氨氧化古菌(AOA)和氨氧化细菌(AOB)丰度对土壤总硝化速率的影响相近,湿化过程中AOB丰度的影响相对增大.
关键词:鄱阳湖干化和干湿转化15N同位素稀释法总氨化速率总硝化速率
Effect of drying-rewetting on soil nitrogen mineralization in Poyang Lake Wetland
WANG Peng1,2

 , YU Xiaofang1,2, XIONG Xiaoying1,2, LIU Junzheng1,2, ZHANG Hua1
, YU Xiaofang1,2, XIONG Xiaoying1,2, LIU Junzheng1,2, ZHANG Hua1 1. Key Laboratory of Poyang Lake Wetland and Watershed Research, Ministry of Education, Jiangxi Normal University, Nanchang 330022;
2. School of Geography and Environment, Jiangxi Normal University, Nanchang 330022
Received 19 April 2019; received in revised from 27 May 2019; accepted 27 May 2019
Abstract: In this study, three typical soils from Carex, Phalaris and Phragmites zones in Poyang Lake wetland were collected, and the soil samples were incubated in laboratory for a month under three moisture levels:30%WHC (maximum water-hold capacity) representing severe drought, 50%WHC representing mild drought and 80%WHC representing suitable moisture condition. Then the three moisture levels were adjusted to 200%WHC to simulate rewetting event. Gross ammoniation rate and gross nitrification rate in wetland soil during the drying-rewetting process were calculated based on the 15N isotope dilution method. In the drying process, the gross ammoniation rate in the soil from the Phragmites zone was the highest, and the gross nitrification rate in the soil from the Carex zone was the highest. Both the gross ammoniation rate and the gross nitrification rate in three typical soils decreased with the increase of drought degree, while the gross nitrification rate dropped more than the gross ammoniation rate under mild drought condition. In addition to the water condition, the gross ammoniation rate was mainly affected by the soil carbon content, and the gross nitrification rate was mainly affected by the pH value. In the rewetting process, the gross ammoniation rate and the gross nitrification rate in soils from the Phalaris and Phragmites zone changed little on the 1st day, then decreased continuously during the 1~5 days. The gross ammoniation rate in the soil from the Carex zone under severe drought maintained an upward trend after rewetting, and that under mild drought only increased on the 1st day after rewetting. All the total nitrification rates in three typical soils decreased significantly on the 1st day after rewetting, and remained low thereafter. The ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) abundance had similar effects on the gross nitrification rate in the drying process, while the AOB abundance had relatively larger effect than the AOA abudance in the wetting process.
Keywords: Poyang Lakedrying-rewetting15N pool dilution methodgross ammoniation rategross nitrification rate
1 引言(Introduction)湿地独特的水土界面环境和较高的生物生产力有利于氮素的快速循环转化, 是氮素重要的储存和转化场所(Jordan et al., 2011).矿化作用是将土壤有机氮转化为无机氮的关键环节, 包括将有机氮转化为NH4+的氨化作用和将NH4+进一步氧化为NO3-的硝化作用.湿地土壤矿化作用产生的NH4+和NO3-可能被微生物或植被吸收固定, 被淋滤进入相邻水体, 或通过硝化、反硝化作用以气体的形式(N2、N2O和NO)释放至大气, 影响陆地-水生生态系统的氮循环过程(Saunders et al., 2001; Zhang et al., 2018).
湿地的水文特性决定了土壤含水量具有明显的季节变化, 进而影响土壤O2含量, 对硝化作用的氧化过程起决定性作用(Butterbach-Bahl et al., 2013).此外, 土壤含水量也通过影响微生物种群的代谢活性以及溶质和酶的扩散速率影响土壤氮矿化速率(Manzoni et al., 2012).干旱环境会导致土壤氮转化速率的降低, 但干化条件下土壤无机氮的产生速率一般大于消耗速率, 会导致土壤无机氮的聚集, 增大雨季来临后的淋失风险(Booth et al., 2005; Dijkstra et al., 2012).早期土壤氮循环的研究常采用净氨化速率和净硝化速率的概念作为可被植物吸收利用的氮通量指标, 但净氮转化速率只代表了若干相互竞争的氮转化过程差值, 不能体现氮的实际转化过程(Xu et al., 2008).Kirkham和Bartholomew(1955)最早提出了15N同位素稀释法, 向土壤加入已知同位素丰度的15N-NH4+或15N-NO3-作为标记, 根据培养前后NH4+、NO3-浓度和15N-NH4+、15N-NO3-丰度变化, 计算总氨化速率和总硝化速率;其基本原理是当某无机N库被高丰度15N标记后, 其它非标记N库中N素的进入会降低其15N丰度, 标记N库N素的输出只改变该N库大小而不改变其15N丰度.15N同位素稀释法是目前唯一可以有效计算总氨化速率和总硝化速率, 即实际氮转化速率的方法(Booth et al., 2005; 程谊等, 2009; Zhang et al., 2015).
鄱阳湖是我国第一大淡水湖, 也是生态多样性丰富的淡水湿地系统.近年来, 鄱阳湖地区的极端干旱频繁发生, 且每年干旱持续时间呈逐渐加长的趋势(刘元波等, 2014).由三峡工程等因素引起的江湖关系变化和鄱阳湖流域气候变化是导致近年来鄱阳湖枯水期水位下降和枯水期延长的主要原因(Zhang et al., 2014; Liu et al., 2017; 胡振鹏等, 2018).枯水期的延长则使鄱阳湖地区容易出现旱涝急转情况(罗蔚等, 2013).本研究对鄱阳湖湿地典型植被土壤进行室内水分控制实验, 模拟不同等级干旱和旱涝急转环境;基于15N同位素稀释法计算土壤氮的总氨化速率和总硝化速率, 分析不同干旱等级下土壤干化-干湿转化过程对土壤氮矿化的影响.
2 材料与方法(Materials and methods)2.1 研究区概况与野外采样本研究选择鄱阳湖国家级自然保护区内的典型湿地(图 1).该湿地受鄱阳湖水位季节性变化影响显著, 枯水期沿坡地至湖面分布藜蒿带、芦苇带、苔草带、虉草带和泥滩带, 大致平行于湖岸成环带状分布;丰水期除藜蒿带外大部分洲滩被淹没.研究区内芦苇、苔草和虉草是鄱阳湖湿地的典型植被(胡振鹏等, 2015), 枯水期完全出露, 雨季发生旱涝急转时可能很快被淹没, 因此选择芦苇带、苔草带和虉草带3处湿地土壤作为研究对象.在枯水期进行采样, 选择植被环带中间高程位置的土壤作为代表(图 1);在每个植被环带中间高程线上采用S型5点采样法, 去除凋落物后, 取表层0~20 cm土壤, 冷藏带回实验室.
图 1(Fig. 1)
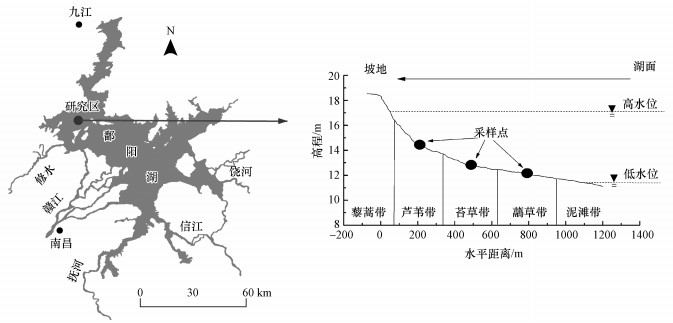 |
| 图 1 研究区位置及采样点示意图 Fig. 1Locations of study area and sampling sites |
2.2 室内水分控制培养与15N稀释实验对采集的3种植被土壤, 过2 mm筛, 测定含水量、最大持水量(WHC)、pH、NH4+、NO3-、总碳(TC)和总氮(TN).含水量通过烘干法测定;WHC采用Fierer等(2002)描述的方法测定;NH4+和NO3-采用KCl溶液提取-分光光度法(HJ 634—2012)测定;土壤pH加水后(1:2.5 (W/V))用pH计测定;TC、TN采用元素分析仪(Vario EL cube, Elementar, 德国)测定.
每个植被带的5个采样点土样充分混合为一个样品, 分别取相当于10 g干土的土样放入432个50 mL培养瓶, 用于水分控制培养实验(图 2).将样品分为3组, 土样先经真空冷冻干燥机(FDU-1200, 东京理化, 日本)去除水分, 然后添加去离子水使土壤含水量分别为30%WHC、50%WHC和80%WHC, 分别模拟重度干旱、轻度干旱和适宜水分环境(参考Hsiao(1973)的研究结论进行干旱等级划分).在培养箱中对3组土壤进行25 ℃恒温避光培养, 用带小孔的Parafilm膜封住瓶口以减弱水分蒸发, 同时保持瓶内外空气流通;每2 d人工添加去离子水, 保持原土壤含水量.1个月后, 添加去离子水调节含水量为200% WHC, 模拟干湿转化环境.不同水分处理的样品同时进行后续15N稀释实验和氨氧化功能基因丰度测定实验.每种处理设置3组平行样.
图 2(Fig. 2)
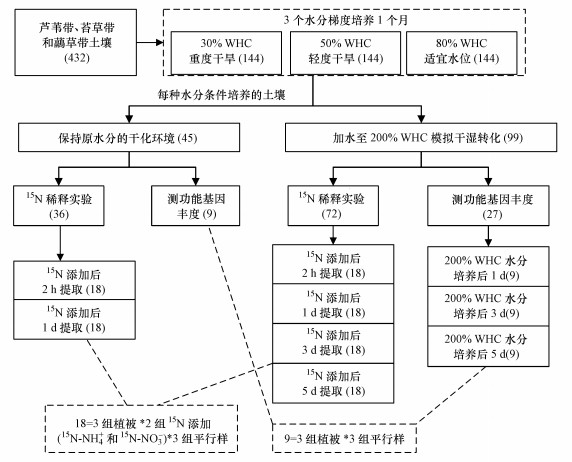 |
| 图 2 湿地土壤的室内水分控制培养实验 (括号内数值为相应样品数) Fig. 2Laboratory culture experiment under different water control for wetland soil |
15N稀释实验参考Hart等(1994)和Cheng等(2012)的实验步骤.一半样品在土样表面均匀加入1 mL 15NH4 NO3(10% access), 使加入N质量相当于10 mg ·kg-1干土, 另外一半加入等量的NH415NO3(10% access).采用2 mol·L-1 KCl提取15N添加后土样的NH4+和NO3-,干化土样分别在15N添加后的2 h、1 d提取;干湿转化土样分别在15N添加后的2 h、1 d、3 d和5 d提取.取5 mL溶液用于测定NH4+和NO3-浓度, 其余溶液采用氨扩散法(Schleppi et al., 2006)分别提取溶液中的NH4+和NO3-, 利用同位素质谱仪(Isoprime100, Elementar, 德国)测定15N-NH4+和15N-NO3-.
在干化-干湿转化过程中(干化0 d和30 d, 湿化1、3和5 d)分别测定土壤氨氧化功能基因AOA-amoA和AOB-amoA丰度.利用E.Z.N.A.? Soil DNA Kit(Omega Bio-tek)提取土壤总DNA.分别利用Arch-amoAF/Arch-amoAR和BamoA1F /BamoA-2R引物(Mao et al., 2011)扩增AOA-amoA和AOB-amoA基因;扩增条件为:95 ℃预变性5 min, 接着40个循环, 包括95 ℃变性15 s, 55 ℃退火30 s, 72 ℃延伸20 s.扩增产物采用荧光定量PCR仪(7500, ABI, 美国)检测定量.
2.3 氮矿化速率计算和数据统计分析总氨化速率和总硝化速率采用Blackburn(1979)提出的计算方法, 该方法在Kirkham和Bartholomew(1955)方法基础上考虑了15N自然丰度, 可以用于低丰度15N添加研究.计算公式如下所示.
 | (1) |
采用单因素方差分析(one-way ANOVA)比较不同组别土壤性质和氮矿化速率差异, 采用Pearson相关系数分析土壤总硝化速率和氨氧化功能基因丰度的相关性.统计分析前对各数据系列进行正态性检验, 对非正态分布的数据进行对数转换.显著性水平设定为 α=0.05.
3 结果(Results)3.1 土壤理化性质所处海拔位置依次升高的虉草带、苔草带和芦苇带3种土壤理化性质具有较大差异(表 1).虉草带土壤具有显著较高的pH值和显著较低的C/N和NH4+含量;芦苇带具有显著高的WHC、TC、C/N值、NH4+和NO3-含量;3种土壤的TN含量没有显著差异, 虉草带和苔草带土壤的WHC、TC和NO3-含量没有显著差异, 苔草带和芦苇带土壤的pH值没有显著差异.
表 1(Table 1)
| 表 1 鄱阳湖湿地虉草带、苔草带和芦苇带土壤理化性质 Table 1 Soil physicochemical properties in Carex zone, Phalaris zone and Phragmites zone in Poyang Lake wetland | ||||||||||||||||||||||||||||||||||||||||
表 1 鄱阳湖湿地虉草带、苔草带和芦苇带土壤理化性质 Table 1 Soil physicochemical properties in Carex zone, Phalaris zone and Phragmites zone in Poyang Lake wetland
| ||||||||||||||||||||||||||||||||||||||||
3.2 15N稀释实验中无机氮浓度和15N丰度15NH4NO3添加实验中, 干化培养添加后2 h~1 d和干湿转化2 h~5 d, 芦苇带土壤NH4+含量显著大于苔草带(p < 0.001), 苔草带显著大于虉草带(p < 0.001)(图 3).芦苇带和苔草带土壤NH4+含量在3种水分下干化-干湿转化过程中均没有明显变化趋势, 在50% WHC和80% WHC水分下略高于30% WHC水分.虉草带土壤NH4+含量在3种水分下差异显著, 30% WHC >50% WHC>80% WHC, 干化添加过程中在30% WHC下呈下降趋势, 在50% WHC和80% WHC下呈上升趋势;干湿转化过程中原30% WHC土壤呈上降趋势, 原50% WHC和原80% WHC土壤呈下降趋势.虉草带土壤15N-NH4原子百分超在干化过程中显著高于苔草带(p=0.034)和芦苇带(p=0.001), 干湿转化过程中快速下降, 逐渐低于苔草带和芦苇带.虉草带15N-NH4原子百分超在3种水分干化过程中差异显著, 50% WHC >30% WHC >80% WHC;干湿转化过程中原30% WHC >原50% WHC >原80% WHC, 但差异未达到显著性.苔草带土壤15N-NH4原子百分超高于芦苇带, 但差异不显著(p=0.082);苔草带和芦苇带土壤15N-NH4原子百分超在3种水分下差异很小, 在培养时间内的动态变化也相近.
图 3(Fig. 3)
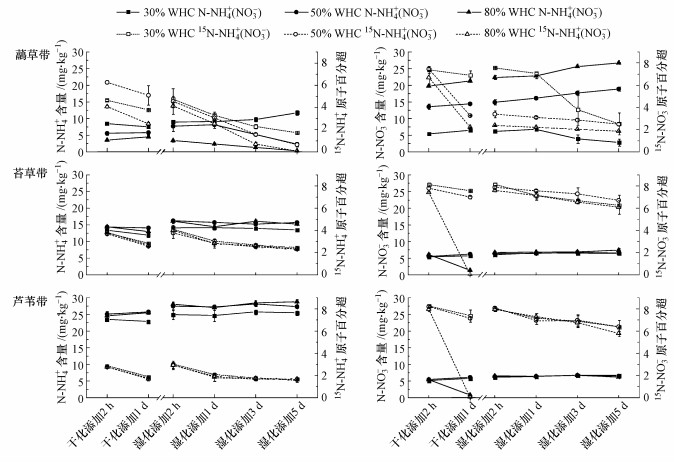 |
| 图 3 15NH4 NO3添加实验中N-NH4+和15N-NH4+丰度以及NH4 15NO3添加实验中N-NO3-和15N-NO3-丰度 Fig. 3Dynamics of N-NH4+ concentration and 15N-NH4+ abundance in 15NH4 NO3 labeled treatment, and N-NO3- concentration and 15N-NO3- abundance in NH4 15NO3 labeled treatment |
NH415NO3添加实验中, 干化培养添加后2 h~1 d和干湿转化2 h~5 d, 3种植被土壤NO3-含量在30% WHC水分下相近, 虉草带土壤NO3-含量在50% WHC和80% WHC水分条件下均显著大于苔草带和芦苇带(p < 0.001)(图 3).虉草带土壤NO3-含量在3种水分下差异显著, 80% WHC >50% WHC >30% WHC, 在干化及干湿转化中都呈上升趋势(30% WHC在干湿转化1 d后呈下降趋势).干化过程中苔草带和芦苇带NO3-含量在30% WHC和50% WHC水分下略有上升, 在80% WHC水分下明显下降.干湿转化过程中苔草带和芦苇带NO3-含量接近, 在3种水分下均无显著差异.虉草带土壤15N-NO3-原子百分超在3种水分干化下差异显著, 30% WHC >50% WHC >80% WHC;干化添加后在50% WHC和80% WHC水分下降明显;湿化添加后在30% WHC下降明显.苔草带和芦苇带15N-NO3-原子百分超数值和变化趋势相近, 干化添加后在80% WHC水分下降明显, 湿化添加后原3种水分土壤都缓慢下降.
3.3 干化-干湿转化过程的土壤总氨化速率和总硝化速率干化培养30 d后, 3种水分条件下总氨化速率均为芦苇带土壤最高(9.08~13.30 mg·kg-1·d-1), 其次为苔草带(2.16~4.85 mg·kg-1·d-1), 虉草带最低(0.68~2.89 mg·kg-1·d-1)(图 4).干化培养后3种植被土壤总氨化速率大体呈现随土壤含水量降低而减小的趋势:虉草带土壤总氨化速率在80% WHC水分下显著高于50% WHC, 在50% WHC水分下显著高于30% WHC;苔草带土壤总氨化速率在80% WHC水分下显著高于30% WHC, 在50% WHC水分下与其它两种水分无显著差异;芦苇带土壤总氨化速率在80% WHC和50% WHC下无显著差异, 但在这两种水分下均显著高于30% WHC.综上可知, 与适宜水分条件(80% WHC)相比, 鄱阳湖湿地3种植被土壤总氨化速率在重度干旱(30% WHC)条件下都显著降低, 但在轻度干旱(50% WHC)条件下只有虉草带土壤总氨化速率显著降低.
图 4(Fig. 4)
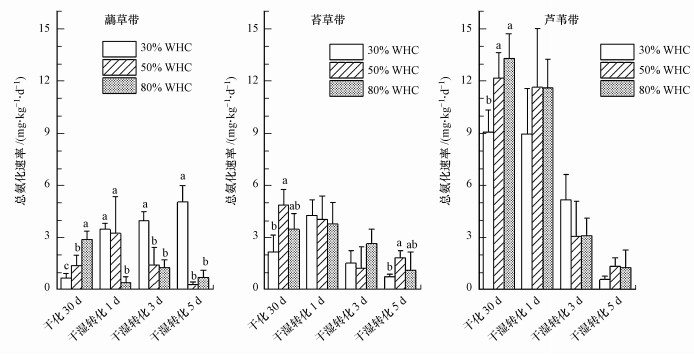 |
| 图 4 干化-干湿转化过程湿地土壤总氨化速率 (不同字母表示该植被土壤总氨化速率在不同水分条件差异显著(p < 0.05)) Fig. 4Gross ammoniation rate during the drying-rewetting process in wetland soil |
干湿转化过程中苔草带和芦苇带土壤总氨化速率动态变化相近(图 4):干湿转化前后总氨化速率接近(苔草带土壤在30%WHC显著上升除外), 干湿转化后1~5 d原3种水分下土壤总氨化速率呈下降趋势;3种水分下土壤总氨化速率差异也不显著(干湿转化5 d苔草带土壤除外).虉草带土壤总氨化速率在干湿转化前后变化明显, 在原30% WHC和50% WHC土壤显著升高, 原80% WHC土壤显著下降;干湿转化后1~5 d, 原30% WHC土壤总氨化速率呈上升趋势, 原50% WHC土壤呈下降趋势, 原80%WHC土壤呈先上升后下降趋势.干湿转化后1~5 d虉草带土壤总氨化速率在不同水分下存在显著差异:干湿转化后1 d原30% WHC和原50% WHC土壤显著大于原80% WHC土壤;3 d和5 d原30% WHC显著大于原50% WHC土壤和原80% WHC土壤.
干化培养30 d后, 3种植被土壤总硝化速率变化范围为0.71~24.05 mg·kg-1·d-1(图 5).50% WHC和80% WHC水分下虉草带土壤总硝化速率均显著高于苔草带和芦苇带(p < 0.001), 30% WHC水分下3种植被土壤间总硝化速率无显著差异.苔草带土壤总硝化速率在50% WHC水分下低于芦苇带, 在80% WHC水分下高于芦苇带, 但差异都不显著.干化培养后3种植被土壤总硝化速率呈现随干旱程度增大而减小的趋势:3种植被土壤总硝化速率在80% WHC水分下均显著高于30% WHC和50% WHC(p < 0.001);虉草带土壤总硝化速率在50% WHC水分下显著高于30% WHC(p < 0.001), 苔草带和芦苇带土壤总硝化速率在50% WHC和30% WHC间均无显著差异.综上可知, 与适宜水分条件(80% WHC)相比, 鄱阳湖湿地3种植被土壤总硝化速率在重度干旱(30% WHC)和轻度干旱(50% WHC)条件下都显著降低;但与轻度干旱相比, 重度干旱下只有虉草带土壤总氨化速率显著降低.
图 5(Fig. 5)
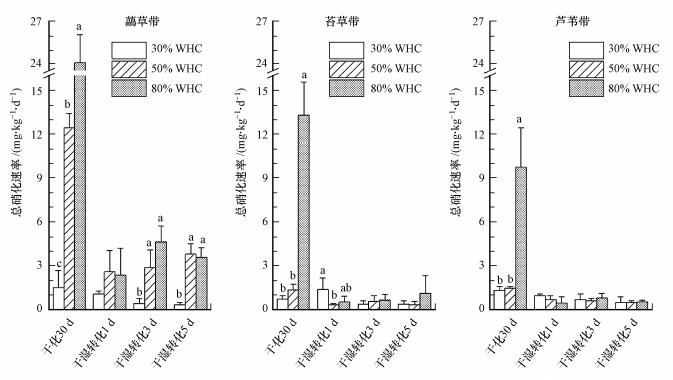 |
| 图 5 干化-干湿转化过程湿地土壤总硝化速率 (不同字母表示该植被土壤总硝化速率在不同水分条件差异显著(p < 0.05)) Fig. 5Gross nitrification rate during the drying-rewetting process in wetland soil |
3种植被土壤在干湿转化后1 d总硝化速率明显降低(原30% WHC苔草带土壤除外), 干湿转化后1~5 d原50% WHC和原80% WHC水分条件的虉草带土壤总硝化速率显著高于苔草带(p < 0.001, p=0.003)和芦苇带(p < 0.001, p=0.002), 原30% WHC水分条件的3种植被土壤总硝化速率无显著差异.干湿转化后1~5 d, 虉草带原50% WHC和原80% WHC土壤总硝化速率在3 d(p=0.019, p=0.002)和5 d(p < 0.001, p=0.001)均显著高于原30% WHC土壤, 苔草带原30% WHC土壤总硝化速率在1 d显著高于原50% WHC土壤(p = 0.048);其它不同水分干化培养土壤在湿化后总硝化速率无显著差异.
3.4 总硝化速率与氨氧化功能基因关系干化培养30 d后, 氨氧化功能基因AOA-amoA和AOB-amoA丰度在严重干旱条件(30%WHC)下都下降, 但在中等干旱条件(50%WHC)下只有AOA-amoA丰度下降(图 6).湿化后AOA-amoA和AOB-amoA丰度动态变化相近:1 d明显下降, 3 d明显上升(30%WHC下苔草带土壤除外), 5 d后在芦苇带上升, 在虉草带和苔草带没有明显规律.干化和干湿转化过程中虉草带土壤AOA-amoA和AOB-amoA丰度均显著大于苔草带和芦苇带, 苔草带和芦苇带间没有显著差异;3种土壤AOB-amoA平均丰度(2.3×108 copies·g-1)高于AOA-amoA(1.0×108 copies·g-1), 但未达到显著性水平.
图 6(Fig. 6)
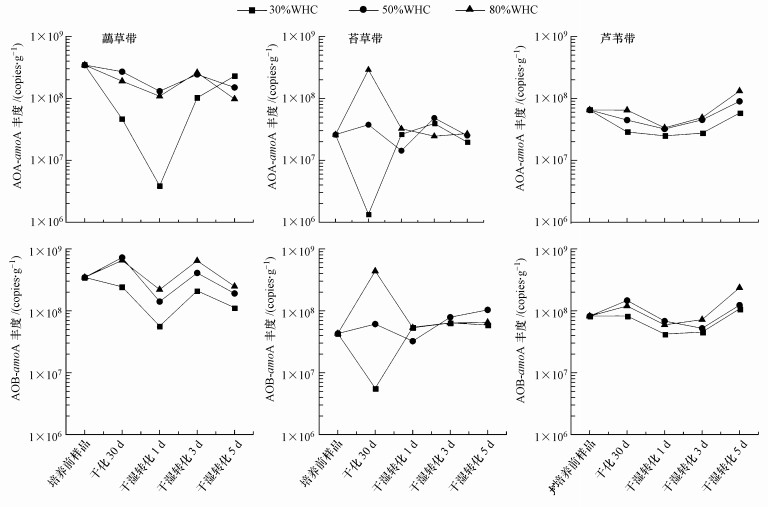 |
| 图 6 干化和干湿转化过程中湿地土壤AOA-amoA和AOB-amoA丰度 Fig. 6AOA-amoA and AOB-amoA abundance during the drying-rewetting process in wetland soil |
干化-干湿转化过程中AOA-amoA和AOB-amoA丰度都与土壤总硝化速率显著正相关, 其中AOB-amoA的相关性大于AOA-amoA(表 2).干化培养后AOB-amoA丰度与土壤总硝化速率的相关系数与AOA-amoA相近, 但干湿转化后AOB-amoA的相关系数明显大于AOA-amoA.干湿转化后AOA-amoA和AOB-amoA丰度与土壤总硝化速率的相关系数在1 d和3 d高于干化土壤, 在5 d低于干化土壤.
表 2(Table 2)
| 表 2 干化和干湿转化过程中湿地土壤氨氧化功能基因丰度与总硝化速率的相关性 Table 2 Correlation coefficients between the abundance of ammonia oxidation functional genes and the gross nitrification rate during the dry-rewetting process in wetland soil | ||||||||||||||||||||||||
表 2 干化和干湿转化过程中湿地土壤氨氧化功能基因丰度与总硝化速率的相关性 Table 2 Correlation coefficients between the abundance of ammonia oxidation functional genes and the gross nitrification rate during the dry-rewetting process in wetland soil
| ||||||||||||||||||||||||
4 讨论(Discussion)本研究发现鄱阳湖湿地3种植被土壤总氨化速率和总硝化速率都随干旱程度增强而降低.土壤有机氮的氨化过程和NH4+的硝化过程都由微生物介导, 土壤水分可作为资源、溶剂和运输介质影响微生物的代谢活性(Schimel, 2018), 从而干旱会降低土壤氮循环转化速率.硝化作用对环境(如温度、水分和酸碱性)变化的响应要比氨化作用更敏感(Zhang et al., 2018);Stark等(1995)研究发现轻度干旱条件下(水势大于-0.6 ×106 Pa)硝化速率的降低主要受NH4+扩散的限制, 重度干旱条件下(水势小于-0.6×106 Pa)硝化速率的降低主要受微生物脱水的限制.本研究也发现在轻度干旱条件下, 由于总硝化速率的降低比总氨化速率更明显(图 3和图 4), 此时总硝化速率降低不仅受到总氨化速率降低的影响(即产生的NH4+减少), 也受到NH4+扩散的限制;重度干旱条件下总氨化速率和总硝化速率都明显降低, 此时二者主要受微生物脱水影响.3种水分条件下总氨化速率均为芦苇带土壤最高, 其次为苔草带, 虉草带最低.芦苇带土壤具有显著较高的TC值(表 1);土壤有机质提供了氮氨化的能量和底物, 而土壤TC与有机质含量密切相关, 所以土壤总氨化速率一般随土壤TC的增加而增加(Booth et al., 2005; Cheng et al., 2019).在50% WHC和80% WHC水分下, 虉草带土壤总硝化速率均显著高于苔草带和芦苇带, 30% WHC水分下3种植被土壤间总硝化速率无显著差异.鲍俊丹等(2011)系统研究了中国典型土壤硝化速率与土壤性质的关系, 发现pH值是影响土壤硝化速率的最主要因素, pH值与土壤最大硝化速率呈显著正相关.虉草带土壤在50%WHC和80%WHC条件下硝化速率明显高于苔草带和芦苇带, 应该与虉草带土壤显著较高的pH值有关(表 2).虉草带土壤在50% WHC和80% WHC水分下总硝化速率较高, 土壤中积累大量NO3-, 由于NO3-相对NH4+不易被土壤吸附(Schwarz et al., 2011), 在干湿转化后虉草带土壤面临较高的氮素淋失风险.
苔草带和芦苇带土壤在湿化转化前后总氨化速率变化较小, 总硝化速率则明显下降;1~5 d总氨化速率不断下降, 总硝化速率维持较低水平.干湿转化过程破坏了土壤团聚体, 暴露更多土壤有机质, 为微生物提供大量营养物质(Fierer et al., 2002), 很多研究发现土壤湿化后氮转化速率增大, 如Dijkstra等(2012)研究了干旱草地土壤在水分输入后氮循环的响应, 发现总氨化速率和总硝化速率在水分输入后的1~3 d快速增大.土壤湿化后微生物活动会很快活跃(1 h内)(Rudaz et al., 1991);Saetre等(2005)发现土壤总氨化速率和总硝化速率在湿化后1 d达到最大, 此后由于湿化后释放的可利用有机碳被消耗导致总氨化速率和总硝化速率明显下降.本研究中苔草带和芦苇带土壤湿化后总氨化速率和总硝化速率没有明显升高可能是由于有机碳在湿化后很快被消耗(湿化后1 d的氮转化速率是通过同时添加15N后2 h~1 d时段计算, 土壤有机碳可能在前2 h被大量消耗).硝化作用通常发生在氧化环境中(Butterbach-Bahl et al., 2013), 受到土壤湿化后还原环境影响, 本研究中的总硝化速率在湿化后明显降低.虉草带原30% WHC和50% WHC土壤总氨化速率在湿化后1 d明显升高, 应该与虉草带土壤显著较低的C/N值(表 1)有关, 微生物对低C/N有机质的分解会导致无机氮的释放(Booth et al., 2005; Cheng et al., 2019).土壤湿化后虉草带原50%WHC和80%WHC土壤总硝化速率明显高于苔草带和芦苇带, 可能与虉草带土壤显著较高的AOA和AOB丰度有关.
鄱阳湖湿地土壤干化-干湿转化过程中, AOA和AOB丰度都对土壤总硝化速率有显著影响.干化过程中, AOA和AOB丰度在严重干旱条件都下降, 在中等干旱条件只有AOA丰度下降, 但AOA和AOB丰度对土壤总硝化速率的影响相近;干湿转化过程中, AOA和AOB丰度都在1 d明显下降, 3 d明显上升, 但AOB丰度的影响相对增大.研究表明碱性土壤中AOB是硝化作用的主导者, 而酸性土壤(pH < 5.5)中AOA是硝化作用的主导者(Prosser et al., 2012; 侯海军等, 2014);本研究中虉草带土壤接近中性, 苔草带和芦苇带土壤呈弱酸性(pH >5.5)(表 1), 从而AOA和AOB对硝化作用都起着重要作用.很多研究表明AOB对水分的响应更敏感(Jie et al., 2015; 刘若萱等, 2015; Marcos et al., 2016).本研究中未发现AOA和AOB丰度对土壤干化及干湿转化的响应有显著差异, 但干旱条件下多数微生物采用休眠的方式躲避不利环境(Jones et al., 2010), 而基于DNA测得的AOA和AOB丰度并不能区分休眠微生物和未休眠微生物(Schimel, 2018).本研究中土壤湿化后AOB丰度对土壤总硝化速率的影响相对增大可能是由于在干湿转化过程中有更多的AOB从休眠中苏醒.
5 结论(Conclusions)1) 土壤干化过程中, 鄱阳湖湿地3种植被土壤总氨化速率和总硝化速率都随干旱程度增强而降低, 轻度干旱条件下总硝化速率的降低更明显.芦苇带土壤总氨化速率最高, 虉草带土壤总硝化速率最高;总氨化速率主要受土壤碳含量影响, 总硝化速率主要受pH值影响.
2) 土壤湿化过程中, 苔草带和芦苇带土壤总氨化速率动态变化相似:湿化后1 d变化较小, 1~5 d不断下降;虉草带重度干旱土壤总氨化速率在湿化后1~5 d呈上升趋势, 轻度干旱土壤只在湿化后1 d明显增大.3种植被土壤湿化后总硝化速率动态变化相似:湿化后1 d明显下降, 1~5 d维持较低水平.
3) 鄱阳湖湿地土壤干化-干湿转化过程中, AOA和AOB丰度都对土壤总硝化速率有显著影响.干化过程中, AOA和AOB丰度对土壤总硝化速率的影响相近, 湿化过程中AOB丰度的影响相对增大.
参考文献
| 鲍俊丹, 石美, 张妹婷, 等. 2011. 中国典型土壤硝化作用与土壤性质的关系[J]. 中国农业科学, 2011, 44(7): 1390–1398.DOI:10.3864/j.issn.0578-1752.2011.07.011 |
| Blackburn T H. 1979. Method for measuring rates of NH4+ turnover in anoxic marine sediments, using a 15N-NH4+ dilution technique[J]. Applied and Environmental Microbiology, 37(4): 760–765. |
| Booth M S, Stark J M, Rastetter E. 2005. Controls on nitrogen cycling in terrestrial ecosystems:a synthetic analysis of literature data[J]. Ecological Monographs, 75(2): 139–157.DOI:10.1890/04-0988 |
| Butterbach-Bahl K, Baggs E M, Dannenmann M, et al. 2013. Nitrous oxide emissions from soils:How well do we understand the processes and their controls?[J]. Philosophical Transactions of the Royal Society B:Biological Sciences, 368(1621): 2033–2045. |
| 程谊, 蔡祖聪, 张金波. 2009. 15N同位素稀释法测定土壤氮素总转化速率研究进展[J]. 土壤, 2009, 41(2): 165–171.DOI:10.3321/j.issn:0253-9829.2009.02.003 |
| Cheng Y, Cai Z, Zhang J, et al. 2012. Soil moisture effects on gross nitrification differ between adjacent grassland and forested soils in central Alberta, Canada[J]. Plant and Soil, 352(1/2): 289–301. |
| Cheng Y, Wang J, Chang S X, et al. 2019. Nitrogen deposition affects both net and gross soil nitrogen transformations in forest ecosystems:A review[J]. Environmental Pollution, 244: 608–616.DOI:10.1016/j.envpol.2018.10.054 |
| Dijkstra F A, Augustine D J, Brewer P, et al. 2012. Nitrogen cycling and water pulses in semiarid grasslands:Are microbial and plant processes temporally asynchronous?[J]. Oecologia, 170(3): 799–808.DOI:10.1007/s00442-012-2336-6 |
| Fierer N, Schimel J P. 2002. Effects of drying-rewetting frequency on soil carbon and nitrogen transformations[J]. Soil Biology and Biochemistry, 34(6): 777–787.DOI:10.1016/S0038-0717(02)00007-X |
| Hart S C, Nason G E, Myrold D D, et al. 1994. Dynamics of gross nitrogen transformations in an old-growth forest:the carbon connection[J]. Ecology, 75(4): 880–891.DOI:10.2307/1939413 |
| 侯海军, 秦红灵, 陈春兰, 等. 2014. 土壤氮循环微生物过程的分子生态学研究进展[J]. 农业现代化研究, 2014, 35(5): 588–594. |
| Hsiao T C. 1973. Plant responses to water stress[J]. Annual Review of Plant Physiology, 24(1): 519–570.DOI:10.1146/annurev.pp.24.060173.002511 |
| 胡振鹏, 傅静. 2018. 长江与鄱阳湖水文关系及其演变的定量分析[J]. 水利学报, 2018, 49(5): 570–579. |
| 胡振鹏, 葛刚, 刘成林. 2015. 鄱阳湖湿地植被退化原因分析及其预警[J]. 长江流域资源与环境, 2015, 24(3): 381–386.DOI:10.11870/cjlyzyyhj201503005 |
| Jie W, Gang L, Xin L, et al. 2015. Differential responses of ammonia-oxidizers communities to nitrogen and water addition in stipa baicalensis steppe, Inner Mongolia, Northern China[J]. Journal of Resources and Ecology, 6(1): 1–11.DOI:10.5814/j.issn.1674-764x.2015.01.001 |
| Jones S E, Lennon J T. 2010. Dormancy contributes to the maintenance of microbial diversity[J]. Proceedings of the National Academy of Sciences of the United States of America, 107(13): 5881–5886.DOI:10.1073/pnas.0912765107 |
| Jordan S, Stoffer J, Nestlerode J. 2011. Wetlands as sinks for reactive nitrogen at continental and global scales:A meta-analysis[J]. Ecosystems, 14(1): 144–155.DOI:10.1007/s10021-010-9400-z |
| Kirkham D, Bartholomew W V. 1955. Equations for following nutrient transformations in soil, utilizing tracer data:Ⅱ[J]. Soil Science Society of America Journal, 19(2): 189–192.DOI:10.2136/sssaj1955.03615995001900020020x |
| 刘若萱, 张丽梅, 白刃, 等. 2015. 模拟条件下土壤硝化作用及硝化微生物对不同水分梯度的响应[J]. 土壤学报, 2015, 52(2): 415–422. |
| 刘元波, 赵晓松, 吴桂平. 2014. 近十年鄱阳湖区极端干旱事件频发现象成因初析[J]. 长江流域资源与环境, 2014, 23(1): 131–138.DOI:10.11870/cjlyzyyhj201401019 |
| Liu Z, Guo S, Guo J, et al. 2017. The impact of Three Gorges Reservoir refill operation on water levels in Poyang Lake, China[J]. Stochastic Environmental Research and Risk Assessment, 31(4): 879–891.DOI:10.1007/s00477-016-1209-7 |
| 罗蔚, 张翔, 邓志民, 等. 2013. 近50年鄱阳湖流域入湖总水量变化与旱涝急转规律分析[J]. 应用基础与工程科学学报, 2013, 21(5): 845–856.DOI:10.3969/j.issn.1005-0930.2013.05.005 |
| Manzoni S, Schimel J P, Porporato A. 2012. Responses of soil microbial communities to water stress:results from a meta-analysis[J]. Ecology, 93(4): 930–938.DOI:10.1890/11-0026.1 |
| Mao Y, Yannarell A C, Mackie R I. 2011. Changes in N-transforming archaea and bacteria in soil during the establishment of bioenergy crops[J]. PloS one, 6(9): e24750.DOI:10.1371/journal.pone.0024750 |
| Marcos M S, Bertiller M B, Cisneros H S, et al. 2016. Nitrification and ammonia-oxidizing bacteria shift in response to soil moisture and plant litter quality in arid soils from the Patagonian Monte[J]. Pedobiologia, 59(1/2): 1–10. |
| Prosser J I, Nicol G W. 2012. Archaeal and bacterial ammonia-oxidisers in soil:the quest for niche specialisation and differentiation[J]. Trends in Microbiology, 20(11): 523–531.DOI:10.1016/j.tim.2012.08.001 |
| Rudaz A O, Davidson E A, Firestone M K. 1991. Sources of nitrous oxide production following wetting of dry soil[J]. FEMS Microbiology Letters, 85(2): 117–124.DOI:10.1111/j.1574-6968.1991.tb04704.x |
| Saetre P, Stark J M. 2005. Microbial dynamics and carbon and nitrogen cycling following re-wetting of soils beneath two semi-arid plant species[J]. Oecologia, 142(2): 247–260.DOI:10.1007/s00442-004-1718-9 |
| Saunders D L, Kalff J. 2001. Nitrogen retention in wetlands, lakes and rivers[J]. Hydrobiologia, 443(1/3): 205–212.DOI:10.1023/A:1017506914063 |
| Schimel J P. 2018. Life in dry soils:effects of drought on soil microbial communities and processes[J]. Annual Review of Ecology, Evolution, and Systematics, 49(1): 409–432.DOI:10.1146/annurev-ecolsys-110617-062614 |
| Schleppi P, Bucher Wallin I, Saurer M, et al. 2006. Citric acid traps to replace sulphuric acid in the ammonia diffusion of dilute water samples for 15N analysis[J]. Rapid Communications in Mass Spectrometry, 20(4): 629–634.DOI:10.1002/rcm.2351 |
| Schwarz M, Oelmann Y, Wilcke W. 2011. Stable N isotope composition of nitrate reflects N transformations during the passage of water through a montane rain forest in Ecuador[J]. Biogeochemistry, 102(1): 195–208. |
| Stark J M, Firestone M K. 1995. Mechanisms for soil moisture effects on activity of nitrifying bacteria[J]. Applied and Environmental Microbiology, 61(1): 218–221. |
| Xu Z, Ward S, Chen C, et al. 2008. Soil carbon and nutrient pools, microbial properties and gross nitrogen transformations in adjacent natural forest and hoop pine plantations of subtropical Australia[J]. Journal of Soils and Sediments, 8(2): 99–105.DOI:10.1065/jss2008.02.276 |
| Zhang J, Cai Z, Müller C. 2018. Terrestrial N cycling associated with climate and plant-specific N preferences:a review[J]. European Journal of Soil Science, 69(3): 488–501.DOI:10.1111/ejss.12533 |
| Zhang J, Müller C, Cai Z. 2015. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils[J]. Soil Biology and Biochemistry, 84: 199–209.DOI:10.1016/j.soilbio.2015.02.028 |
| Zhang Q, Ye X, Werner A D, et al. 2014. An investigation of enhanced recessions in Poyang Lake:Comparison of Yangtze River and local catchment impacts[J]. Journal of Hydrology, 517: 425–434.DOI:10.1016/j.jhydrol.2014.05.051 |
