 , 姚静波, 王明新
, 姚静波, 王明新
 , 朱颖一, 王城晨
, 朱颖一, 王城晨常州大学环境与安全工程学院, 常州 213164
收稿日期: 2017-12-25; 修回日期: 2018-02-03; 录用日期: 2018-02-03
基金项目: 江苏省产学研合作研究项目(No.BY2015027-08);江苏省"六大人才高峰"培养对象资助项目(No.JNHB-003);江苏省普通高校专业学位研究生实践创新计划项目
作者简介: 荀志祥(1991-), 男, E-mail:821194824@qq.com
通讯作者(责任作者): 王明新, E-mail:wmxcau@163.com
摘要: 采用响应面法实验设计研究了超声辅助EDDS/EGTA淋洗对Cu、Zn、Pb和Cd等不同重金属的洗脱效果和对残留重金属化学形态分布的影响,以超声功率、初始pH、EDDS投加量、EGTA投加量作为考察对象,以各重金属去除率及其环境风险削减率为响应值进行了模拟和优化.结果表明,EDDS对Pb的洗脱效率最高,Cu、Zn次之,Cd最低;EGTA对Cd的洗脱效率最高,Pb、Zn次之,Cu最低;超声功率对Cu、Pb去除强化效果明显,对Cd影响小,对Zn无显著影响;初始pH值为酸性时,Cu和Zn去除率高,碱性时Pb去除率较高,Cd去除率有所下降.EDDS与EGTA投加量及EGTA投加量与初始pH值对环境风险总削减率存在交互作用.超声辅助EDDS/EGTA对可还原态重金属洗脱效果较好,但容易导致弱酸提取态Cu和Pb残留率有所升高.响应面优化结果表明,当EDDS和EGTA投加量分别为重金属总摩尔数的1.92和2.56倍、超声功率为600 W、淋洗液初始pH值为5.27时,环境风险总削减率达到77.58%,与模拟值相近,模型具有较好的模拟和预测能力.
关键词:土壤重金属淋洗形态环境风险响应面法
Effects of ultrasound-assisted EDDS/EGTA washing on specification and environmental of heavy metals in soil and optimization by response surface method
XUN Zhixiang
 , YAO Jingbo, WANG Mingxin
, YAO Jingbo, WANG Mingxin
 , ZHU Yingyi, WANG Chengchen
, ZHU Yingyi, WANG Chengchen School of Environmental & Safety Engineering, Changzhou University, Changzhou 213164
Received 25 December 2017; received in revised from 3 February 2018; accepted 3 February 2018
Supported by the University-Industry Cooperation Program of Jiangsu Province (No. BY2015027-08), the Training Object Financing Project of "Six Major Talent Summit" in Jiangsu Province (No.JNHB-003) and the Project of Practical Innovation of Professional Degree Graduate Students in Jiangsu Province
Biography: XUN Zhixiang(1991—), male, E-mail:821194824@qq.com
*Corresponding author: WANG Mingxin, E-mail:wmxcau@163.com
Abstract: The response surface method was used to design an experiment and investigate the effect of ultrasound-assisted EDDS/EGTA washing on the removal efficiency of heavy metals including Cu, Zn, Pb and Cd in soil and the specification of residual heavy metals. Taking the ultrasonic power, initial pH, EDDS dose and EGTA dose as the factors, and the removal rate of heavy metals and environmental risk as the response values, the relationship between response values and factors was fitted and optimized. The results showed that the removal efficiency of Pb by EDDS was the highest, following by Cu and Zn, and Cd was the lowest. The EGTA showed the highest removal rate of Cd, following by Pb, Zn and Cu. The ultrasonic power showed significant removal effect on Cu and Pb, little effect on Cd, and no significant effect on Zn. The removal rates of Cu and Zn were high under acid condition. Under alkaline condition, the removal rate of Pb was high while the removal rate of Cd decreased. The ultrasound-assisted EDDS/EGTA showed high washing efficiency of heavy metals in reducible state, but may lead to the increase of copper and lead in weak acid extractable state. EDDS and EGTA, EGTA and initial pH both showed interaction effect on reduction rate of the total environmental risk. The response surface optimization results showed that the optimal washing condition was as following:the EDDS dose was 1.92, the EGTA dose was 2.56, the ultrasonic power was 600 W and the initial pH was 5.27, the reduction rate of total environmental risk was 77.58%, which was similar to the simulated value. Therefore, the model has good fitting and predicting ability.
Key words: soilheavy metalwashingspeciationenvironmental riskresponse surface methodology
1 引言(Introduction)随着工业化和城市化的发展, 重金属污染成为日益严重的环境问题之一.城市建设和工厂外迁遗留下大批重金属污染场地(Shao et al., 2016;黄文华等, 2016), 其土壤存在严重的环境风险, 迫切需要快速有效的修复技术.重金属污染土壤修复技术主要有固定/稳定化、热处理、氧化还原、淋洗修复技术等(李玉双等, 2011;Oves et al., 2016).其中, 淋洗技术能够快速、高效地洗脱土壤中的重金属(Torres et al., 2012), 适用于污染集中、修复面积小、污染严重的区域(李实等, 2014;Elghdalgren et al., 2009), 因而成为重金属污染土壤修复的主要技术之一(孙涛等, 2015).
化学螯合剂中乙二胺四乙酸(Ethylenediaminetetraacetic acid, 简称EDTA)因其对土壤中多种重金属具有较好的螯合能力和洗脱效果而得到了广泛的研究和应用, 但EDTA难于生物降解, 在土壤中的半衰期较长, 残留时间较长, 因此许多****采用容易生物降解的螯合剂如N, N′-乙二胺二琥珀酸(N, N′-ethylenediaminedisuccinic acid, 简称EDDS)、谷氨酸二乙酸四钠(N, N-Bis(carboxymethyl)-L-glutamic acid tetrasodium salt, 简称GLDA)、乙二醇双(2-氨基乙基醚)四乙酸(Ethylenebis(oxyethylenenitrilo)tetraacetic acid, 简称EGTA)等来替代或部分替代EDTA, 但这些替代螯合剂往往仅对某一种或几种重金属具有较好的洗脱效果, 因此需要根据土壤中重金属种类选择适宜的螯合剂进行混合淋洗才能取得较好的效果.此外螯合剂的使用会转变土壤中重金属的存在形态(Y?ld?r?m et al., 2016), 需要对其产生的环境风险进行评价和管控.因为重金属污染风险不仅取决于重金属的总浓度(谭业华等, 2011), 也与重金属的化学形态及其生物可利用性有关(Lim et al., 2008;Kwon et al., 2001), 重金属的总浓度并不能为判断重金属的生物可利用性和毒性提供足够的信息量(Sundaray et al., 2011).目前大部分相关研究主要关注淋洗剂及淋洗条件对不同重金属的洗脱效果, 很少关注淋洗剂对土壤中不同形态重金属转化的影响, 也很少关注淋洗对土壤重金属环境风险的削减效果(Zang et al., 2013).
本研究以含Cu、Zn、Pb和Cd等多金属污染土壤为供试对象, 采用响应面中心组合设计方法, 模拟超声辅助EDDS/EGTA混合淋洗剂对土壤重金属的洗脱效果及对残留重金属形态分布的影响.采用综合考虑了重金属残留量、生物有效性和生理毒性的环境风险削减率为响应值, 拟合重金属去除率和环境风险削减率与淋洗条件的关系并进行拟合和优化, 旨在为利用超声强化可生物降解螯合剂快速淋洗修复重金属污染土壤提供科学依据.
2 材料和方法(Materials and methods)2.1 试验材料供试土壤取自江苏省常州市武进区科教城周边菜地清洁土壤, 经自然风干后, 去除碎石及杂草, 研磨过5 mm尼龙筛, 加入一定量的重金属溶液染土并静置1 a.所用试剂分别为:CuSO4·5H2O、ZnSO4·7H2O、Pb(NO3)2, CdCl2·5H2O.四分法取部分土样研磨后, 过100目筛, 供重金属全量分析用.土壤重金属含量及形态见表 1.
表 1(Table 1)
| 表 1 供试土壤的重金属含量 Table 1 Heavy metal concentration of the test soil | ||||||||||||||||||||||||||||||
表 1 供试土壤的重金属含量 Table 1 Heavy metal concentration of the test soil
| ||||||||||||||||||||||||||||||
2.2 实验设计前期实验结果和前人文献报道表明EDDS对Zn、Pb有较好的洗脱效果, EGTA对Cd有很强的螯合能力(薛腊梅等, 2013;伊雪等, 2014), 超声功率和初始pH对淋洗效果有一定影响(张春雷等, 2014).因此本文选取EDDS投加量、EGTA投加量、超声功率、初始pH等4个对重金属洗脱效果影响较大的因素为考察对象, 每个因素设置3个水平, 采用响应面优化法设计实验处理, 各因素与水平设计如表 2所示.响应面法(Response Surface Methodology, RSM)是一种综合实验设计和数学建模的优化方法, 通过对部分代表性点进行实验, 拟合出各因素与实验结果之间的函数关系, 建立连续变量曲面模型, 确定实验因素及其交互作用在工艺过程中对响应值的影响, 对实验条件进行优化并预测实验结果.同传统的单因素或正交实验相比, 响应面法具有实验次数少、准确率高、直观性强和预测性好等优点(Ahmad et al., 2005;Wang et al., 2011).
表 2(Table 2)
| 表 2 实验因素与水平设计 Table 2 Experimental factors and level design | |||||||||||||||||||||||||
表 2 实验因素与水平设计 Table 2 Experimental factors and level design
| |||||||||||||||||||||||||
2.3 重金属含量及化学形态分析将污染土壤混合均匀后取适量土样研磨过100目筛备用.每个处理称取1 g污染土样于100 mL离心管中, 加入一定量的淋洗剂, 超声淋洗40 min, 然后经4000 r·min-1离心20 min后, 取上清液, 用火焰原子吸收分光光度法测定金属离子的含量.采用连续提取形态分析法(BCR)(Ruban et al., 1999)测定各重金属化学形态, 称取1 g土样放入100 mL离心管中, 将重金属形态分为弱酸提取态、可还原态、可氧化态和残渣态4种形态, 采用火焰原子吸收分光光度法测定不同形态Cu、Zn、Pb和Cd的质量浓度.
2.4 环境风险评价风险评价编码(Risk Assessment Code, 简称RAC)是重金属风险评价的一种方法.根据重金属的化学形态对风险等级进行分类风险标准如表 3所示(Perin et al., 1985).潜在生态风险指数法(Potential Ecological Risk Index, 简称RI)是由瑞典****Hakanson(1980)建立的评价重金属污染及生态风险性的方法.RAC忽略了不同重金属的毒性反应因子, RI考虑了重金属的毒性和总含量, 但忽略了生物有效性的影响.
表 3(Table 3)
| 表 3 模糊毒性指数?值 Table 3 Values of ? | ||||||||||||||||||
表 3 模糊毒性指数?值 Table 3 Values of ?
| ||||||||||||||||||
本文采用基于模糊毒性指数改进的潜在生态风险指数法(Modified Potential Ecological Risk Index, 简称MRI)对淋洗后的土壤进行环境风险评价(Zhu et al., 2012;胡延彪等, 2016).该方法采用RAC对RI进行修正, 计算公式如下所示.
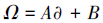 | (1) |
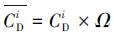 | (2) |
 | (3) |
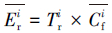 | (4) |
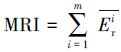 | (5) |
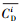
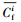
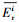
淋洗后的土壤重金属环境风险削减率采用下式计算.
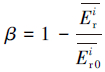 | (6) |
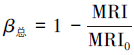 | (7) |
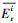
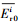
表 7(Table 7)
| 表 7 不同淋洗条件下重金属环境风险削减率 Table 7 Reduction rates of heavy metal under different washing conditions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 7 不同淋洗条件下重金属环境风险削减率 Table 7 Reduction rates of heavy metal under different washing conditions
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2.5 模型拟合和优化采用Design-expert 8.0和二次多项式模型对重金属去除率、MRI削减率与淋洗条件之间的数量关系进行逐步回归拟合, 分析模型的显著性及可靠性, 得到回归方程和优化条件, 对优化结果进行验证.
3 结果与讨论(Results and discussion)3.1 各重金属去除率及其形态分布变化分析采用Design-expert 8.0的Box-Behnken进行实验设计得到29个处理, 用火焰原子吸收分光光度法测定淋洗液中金属离子含量后, 经计算得到Cu、Zn、Pb和Cd等重金属的去除率(表 4), 对淋洗后的土壤采用BCR进行连续提取测定, 经计算得到Cu、Zn、Pb和Cd等重金属各不同形态的残留率(表 5).各不同形态重金属的残留率为淋洗后该形态重金属残留量占淋洗前该重金属总量的百分比.
表 4(Table 4)
| 表 4 Box-Behnken design实验设计及各淋洗条件下各重金属去除率 Table 4 Box-Behnken design and heavy metal removal efficiency under different washing conditions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 4 Box-Behnken design实验设计及各淋洗条件下各重金属去除率 Table 4 Box-Behnken design and heavy metal removal efficiency under different washing conditions
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 5(Table 5)
| 表 5 不同形态重金属残留率 Table 5 The retention percentages of heavy metal in different state | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 5 不同形态重金属残留率 Table 5 The retention percentages of heavy metal in different state
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cu去除率在10.73%~82.84%之间, 处理7的Cu去除率最高, 达到82.84%.供试土壤淋洗前弱酸提取态Cu占36.22%, 淋洗后部分处理土壤中弱酸提取态Cu残留率有所升高, 可能是部分可还原态Cu转化成了弱酸提取态Cu(Finzgar et al., 2007).魏岚等(2010)研究表明EDDS等螯合剂会使土壤中弱酸提取态重金属含量显著增加而还原态显著下降, 与本研究结果相似.供试土壤中可还原态Cu占40.96%, 经不同淋洗条件淋洗后土壤中残留率呈降低趋势, 可能是一部分可还原态Cu在螯合剂的作用下被洗脱进入淋洗液, 另有一部分转化成了弱酸提取态Cu, 这与伊雪等(2014)的研究结果相似.供试土壤中可氧化态Cu和残渣态Cu较少, 因此各不同处理条件下以上2种形态Cu残留率的变化较小.
Zn去除率在9.33%~67.41%之间, 处理27的Zn去除率最高, 达到67.41%.淋洗前土壤中弱酸提取态Zn占39.94%, 经不同淋洗处理后残留率在16.11%~33.13%之间.淋洗前土壤中可还原态Zn占40.8%, 淋洗后最高残留率为38.42%, 最低为11.08%, 不同淋洗条件下残留率差异很大.淋洗前土壤中可氧化态Zn含量较低, 仅占4.32%, 淋洗后各处理中可氧化态Zn残留率在0.11%~3.45%之间.淋洗前土壤中残渣态Zn占14.94%, 处理11、16、27中残渣态Zn残留率在1%以下, 处理17、25、29中的残留率则有所增加, 可能是由于其它形态的Zn转化成了残渣态Zn.
Pb去除率在4.2%~94.32%之间, 处理3的Pb去除率最高, 达到94.32%.供试土壤淋洗前仅有4.06%的Pb以弱酸提取态存在, 淋洗后多个处理中弱酸提取态Pb残留率有所增加.供试土壤淋洗前Pb主要以可还原态存在, 占78.86%, 淋洗后各处理的可还原态Pb残留率大部分在30.0%以下, 最低为1.70%, 这主要是由于可还原态Pb较易被洗脱, 其中有部分可还原态Pb可能转化为弱酸提取态Pb, 导致弱酸提取态Pb有所增加.各处理可氧化态Pb残留率与淋洗前相近, 残差态Pb残留率则存在较大差异.
Cd去除率在33.04%~79.13%之间, 处理7的Cd去除率最高, 达到79.13%.供试土壤淋洗前弱酸提取态Cd占40.34%, 淋洗后残留率在8.35%~21.69%之间.供试土壤淋洗前可还原态Cd占24.41%, 淋洗后残留率在4.9%~24.03%之间, 呈下降趋势.淋洗前可氧化态Cd含量较少, 淋洗后土壤中残留率相近且差异相对较小.淋洗前土壤中残渣态Cd占31.31%, 经不同淋洗条件淋洗后, 残留率降至较低水平.
3.2 土壤重金属去除率与淋洗条件的关系拟合分别将Cu、Zn、Pb、Cd的去除率导入Design-expert 8.0中, 选择二次多项式采用逐步回归法进行拟合, 得到4个模型, 模型的p值均小于0.0001, 表明回归模型极为显著(陈志良等, 2015), 失拟项均不显著(失拟项p值> 0.05), 模型相关性好.其相应的模型回归方程和方差分析如表 6所示.其它条件取中值的条件下, 不同淋洗条件变化对响应值的影响见图 1.
表 6(Table 6)
| 表 6 模型回归方程及方差分析 Table 6 Model regression equation and analysis of variance | ||||||||||||||||||||||||||||||
表 6 模型回归方程及方差分析 Table 6 Model regression equation and analysis of variance
| ||||||||||||||||||||||||||||||
图 1(Fig. 1)
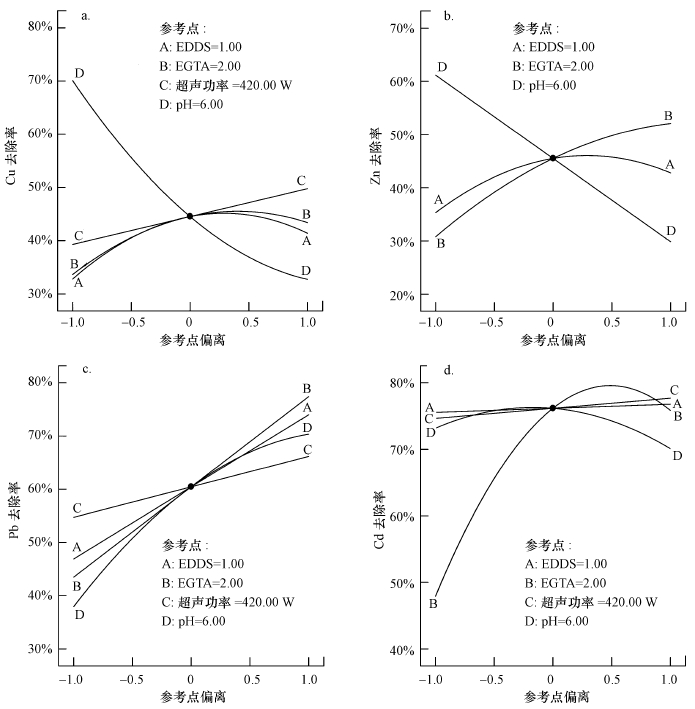 |
| 图 1 重金属去除率与淋洗条件的关系 Fig. 1The relationship between heavy metal removal rates and various factors |
图 1反映了其它实验因素取参考点(中值)时, 淋洗条件对各重金属去除率的影响程度.横坐标中-1和1分别代表最小值和最大值, -0.5和0.5分别代表最小值与中值的均值、中值与最大值的均值.EDDS和EGTA对Cu的洗脱效果相近, Cu去除率对EDDS和EGTA投加量的依赖性较小, 这可能是由于Cu的稳定常数为18.4, 高于其他金属, 在螯合剂剂量较小时有较大的竞争优势(Hai et al., 2008);酸性条件下有利于Cu的洗脱, 碱性条件下对Cu的洗脱有较大的抑制作用(图 1a).EDDS和EGTA对Zn的去除都有促进作用, 但EDDS作用效果小于EGTA.超声功率对Zn的去除影响较小.Zn在酸性条件下去除率高, 当初始pH为碱性时, Zn去除率大幅下降(图 1b).Pb去除率随EDDS投加量、EGTA投加量、超声功率、初始pH值的增大而升高, 其影响程度为EGTA投加量>EDDS投加量>初始pH值>超声功率(图 1c).Cd的去除效果主要受EGTA投加量的影响, 随着EGTA浓度的增加Cd去除率迅速升高, 但投加量过高也会使Cd的去除率有所下降(图 1d).
3.3 土壤重金属环境风险削减与淋洗条件的关系拟合通过基于模糊毒性指数改进的潜在生态风险指数法计算公式及环境风险削减率公式计算得到Cu、Zn、Pb和Cd等4种重金属的环境风险削减率和重金属环境风险总削减率如表 7所示.
采用Design-Experts 8.0的逐步回归方法拟合各重金属环境风险削减率与淋洗条件之间的关系, 以及重金属环境风险总削减率与淋洗条件之间的关系, 得到如表 8所示的二阶多项式模型及其方差分析结果.
表 8(Table 8)
| 表 8 模型拟合及方差分析 Table 8 Model fitting and variance analysis | |||||||||||||||||||||||||||||||||||
表 8 模型拟合及方差分析 Table 8 Model fitting and variance analysis
| |||||||||||||||||||||||||||||||||||
各模型F值较大, p值均小于0.0001, 回归模型极为显著, 失拟项均不显著(失拟项p值> 0.05), 表明回归模型正确, 实验方法可靠.由于采用逐步回归方法进行模型拟合, 确保进入回归模型中的实验因素均为显著(p < 0.05).模型的决定系数R2都在0.90以上, 说明相关性好, 模型可靠性强.
图 2分别为Cu、Zn、Pb、Cd环境风险削减率及环境风险总削减率的观察值与模拟值散点图, 散点基本分布在直线上或是两侧, 表明观察值与模拟值相近, 各模型的拟合效果较好, 反映了各模型均具有良好的预测性和稳定性.
图 2(Fig. 2)
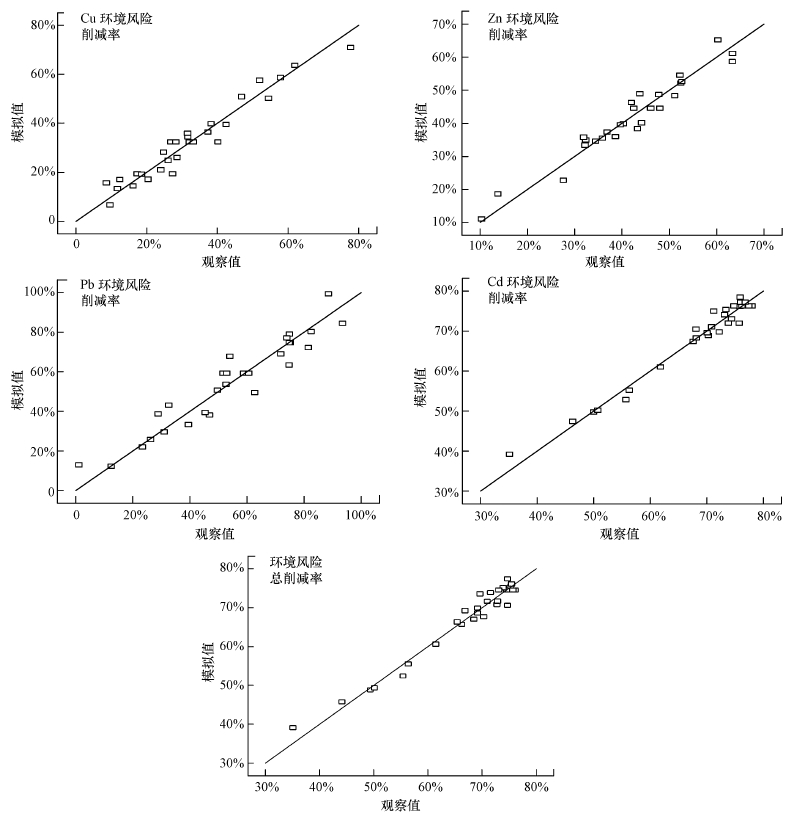 |
| 图 2 观察值与模拟值相关性图 Fig. 2Correlation diagram between observed and simulated values |
3.4 因素互作的响应面分析图 3a反映超声功率为420 W、初始pH值为6时, EDDS投加量与EGTA投加量的交互作用对环境风险总削减率的影响.当EGTA投加量处于较低位置时, 环境风险总削减率随EDDS投加量的增加而逐渐升高, 但当EGTA投加量较高时, 随着EDDS投加量的增加, 环境风险总削减率有所下降.当EDDS投加量一定时, 环境风险总削减率随EGTA投加量的增加先升高后趋平, 最后又略微下降.这可能是由于EGTA投加量过高后, Cd去除率有所下降, 而Cd的环境风险较大.当然出现这样的结果是EDDS和EGTA对Cu、Zn、Pb和Cd综合作用影响所致, 但Cd的毒性系数较高, 土壤背景值较低, 潜在生态风险指数较大, 因此可能起主导作用.
图 3(Fig. 3)
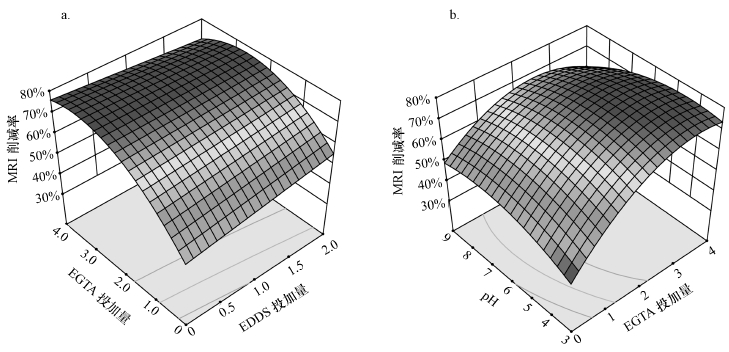 |
| 图 3 MRI削减率响应曲面分析图 Fig. 3Response surface analysis of MRI reduction rate |
图 3b反映EDDS投加量为1、超声功率为420 W时, EGTA投加量与淋洗液初始pH值的交互作用对环境风险总削减率的影响.当初始pH一定且较低时, 环境风险总削减率随EGTA投加量的增加先不断升高后趋于平缓.当初始pH值一定且较大时, 环境风险总削减率随EGTA投加量的增加先升高后下降;当EGTA投加量一定且浓度较低时, 环境风险总削减率随着初始pH值的升高而升高.当EGTA投加量一定且浓度较高时, 环境风险总削减率随着初始pH值的升高而降低.
3.5 最优处理条件及验证以环境风险总削减率为响应值, 采用Design-expert 8.0优化得到的最优淋洗条件为:EDDS投加量为1.92、EGTA投加量为2.56、超声功率为600 W, pH值为5.27, 环境风险总削减率模拟值为78.68%.
根据以上模拟结果进行验证实验, 得到Cu、Zn、Pb和Cd等4种重金属的去除率及环境风险削减率如表 9所示.环境风险总削减率为77.58%, 与模拟值相近, 表明模型具有较好的模拟和预测能力, 因而具有一定的应用价值.
表 9(Table 9)
| 表 9 验证实验条件下重金属去除率及其环境风险削减率 Table 9 Reduction rates heavy metals and environmental risk under validation experiment conditions | |||||||||||||||
表 9 验证实验条件下重金属去除率及其环境风险削减率 Table 9 Reduction rates heavy metals and environmental risk under validation experiment conditions
| |||||||||||||||
4 结论(Conclusions)1) 超声辅助EDDS/EGTA复合淋洗对Cu、Zn、Pb和Cd均有较好的去除效果, 其中Pb去除率可达90%以上, Cd去除率和环境风险总削减率可达近80%.
2) 淋洗条件对残留重金属形态分布有显著影响, 可还原态重金属洗脱较为显著, 部分处理中弱酸提取态Cu和Pb重金属残留率有所增加, 可能是由于其它形态重金属尤其是还原态重金属转化成弱酸提取态.
3) 采用二次多项式进行逐步回归拟合了MRI削减率与超声强度、初始pH、EDDS投加量和EGTA投加量等4个淋洗条件之间的关系, 各模型均极为显著, 拟合效果较好, 可靠性较高.
4) 以MRI削减率为响应值优化得到的淋洗方案对生理毒性较高的Pb和Cd的洗脱率较高, 各重金属去除率和环境风险总削减率模拟值与验证实验结果相近, 表明模型具有较好的模拟和预测能力.
参考文献
| Ahmad A L, Ismail S, Bhatia S. 2005. Optimization of coagulation-flocculation process for palm oil mill effluent using response surface methodology[J]. Environmental Science & Technology, 39(8): 2828–2834. |
| 陈志良, 雷国建, 苏耀明, 等. 2015. 茶皂素与EDTA淋洗对土壤中铅、锌形态的影响[J]. 生态环境学报, 2015, 24(8): 1394–1398. |
| Delgado J, Barba-Brioso C, Nieto J M, et al. 2011. Speciation and ecological risk of toxic elements in estuarine sediments affected by multiple anthropogenic contributions (Guadiana saltmarshes, SW Iberian Peninsula):I. Surficial sediments[J]. Science of the Total Environment(19): 3666–3679. |
| Elghdalgren K, Arwidsson Z, Camdzija A, et al. 2009. Laboratory and pilot scale soil washing of PAH and arsenic from a wood preservation site:Changes in concentration and toxicity[J]. Journal of Hazardous Materials, 172(2): 1033–1040. |
| Finzgar N, Lestan D. 2007. Multi-step leaching of Pb and Zn contaminated soils with EDTA[J]. Chemosphere, 66(5): 824–832.DOI:10.1016/j.chemosphere.2006.06.029 |
| Hai W U, Wang J X, Huang J M, et al. 2008. Optimization of Cudrania extractum tablets formulation by central composite design-response surface methodology[J]. Fudan University Journal of Medical Sciences, 35(3): 363–370. |
| Hakanson L. 1980. An ecological risk index for aquatic pollution control. a sedimentological approach[J]. Water Research, 14(8): 975–1001.DOI:10.1016/0043-1354(80)90143-8 |
| 胡延彪, 李忠武, 黄金权, 等. 2016. 湘江长沙段洲滩菜园土壤重金属潜在生态风险评价[J]. 安全与环境学报, 2016, 16(1): 354–358. |
| 黄文华, 郭鸿. 2016. 工业废弃地景观更新模式研究[J]. 工业建筑, 2016, 46(8): 69–72. |
| Kwon Y T, Lee C W. 2001. Ecological risk assessment of sediment in wastewater discharging area by means of metal speciation[J]. Microchemical Journal, 70(3): 255–264.DOI:10.1016/S0026-265X(01)00122-9 |
| Lim H S, Lee J S, Chon H T, et al. 2008. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au-Ag mine in Korea[J]. Journal of Geochemical Exploration, 96(2/3): 223–230. |
| 李实, 张翔宇, 潘利祥. 2014. 重金属污染土壤淋洗修复技术研究进展[J]. 化工技术与开发, 2014, 2014(11): 27–31.DOI:10.3969/j.issn.1671-9905.2014.11.009 |
| 李一蒙, 马建华, 刘德新, 等. 2015. 开封城市土壤重金属污染及潜在生态风险评价[J]. 环境科学, 2015, 3(3): 1037–1044. |
| 李玉双, 胡晓钧, 孙铁珩, 等. 2011. 污染土壤淋洗修复技术研究进展[J]. 生态学杂志, 2011, 30(3): 596–602. |
| Oves M, Khan M S, Zaidi A, et al. 2012. Soil Contamination, Nutritive Value, and Human Health Risk Assessment of Heavy Metals:An Overview[M]. Springer Vienna: 1–27. |
| Perin G, Craboledda L, Lucchese L, et al. 1985. Heavy metal speciation in the sediments of Northern Adriatic Sea. A new approach for environmental toxicity determination[C]. Heavy Metals in the Environment, 454-456 |
| Ruban V, Lópezsánchez J F, Pardo P, et al. 1999. Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediment[J]. Journal of Environmental Monitoring Jem, 1(1): 51–56.DOI:10.1039/a807778i |
| Shao D, Zhan Y, Zhou W, et al. 2016. Current status and temporal trend of heavy metals in farmland soil of the Yangtze River Delta Region:Field survey and meta-analysis[J]. Environmental Pollution, 219: 329–336.DOI:10.1016/j.envpol.2016.10.023 |
| Sundaray S K, Nayak B B, Lin S, et al. 2011. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-A case study:Mahanadi basin, India[J]. Journal of Hazardous Materials, 186(2/3): 1837–1846. |
| 孙涛, 陆扣萍, 王海龙. 2015. 不同淋洗剂和淋洗条件下重金属污染土壤淋洗修复研究进展[J]. 浙江农林大学学报, 2015, 32(1): 140–149.DOI:10.11833/j.issn.2095-0756.2015.01.021 |
| 谭业华, 魏建和, 陈珍, 等. 2011. 海南槟榔园土壤重金属含量分布与评价[J]. 中国环境科学, 2011, 31(5): 815–819. |
| Torres L G, Lopez R B, Beltran M. 2012. Removal of As, Cd, Cu, Ni, Pb and Zn from a highly contaminated industrial soil using surfactant enhanced soil washing[J]. Physics and Chemistry of the Earth, 37-39(3): 30–36. |
| Wang J P, Chen Y Z, Wang Y, et al. 2011. Optimization of the coagulation-flocculation process for pulp mill wastewater treatment using a combination of uniform design and response surface methodology[J]. Water Research, 45(17): 5633–5640.DOI:10.1016/j.watres.2011.08.023 |
| 魏岚, 陈亚华, 钱猛, 等. 2006. 可降解螯合剂EDDS诱导植物修复重金属污染土壤的潜力[J]. 南京农业大学学报, 2006, 29(2): 33–38. |
| Y?ld?r?m G, Tokal?o?lu ?. 2016. Heavy metal speciation in various grain sizes of industrially contaminated street dust using multivariate statistical analysis[J]. Ecotoxicol Environ Saf, 128: 266–266.DOI:10.1016/j.ecoenv.2015.12.041 |
| 尹雪, 陈家军, 吕策. 2014. 螯合剂复配对实际重金属污染土壤洗脱效率影响及形态变化特征[J]. 环境科学, 2014, 35(2): 733–739. |
| Zang S Y, Wang Z K, De M A. 2013. Heavy metal pollution in the soil in-situ and the concerned evaluation of the mineral storage yards of Tianjin Port Harbor[J]. Journal of Safety and Environment, 13(4): 146–150. |
| 中国环境监测总站. 1990. 中国土壤元素背景值[M]. 北京: 中国环境科学出版社. |
| 周智全, 张玉歌, 徐欢欢, 等. 2016. 化学淋洗修复重金属污染土壤研究进展[J]. 绿色科技, 2016(24): 12–15. |
| Zhu H N, Yuan X Z, Zeng G M, et al. 2012. Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index[J]. Transactions of Nonferrous Metals Society of China, 22(6): 1470–1477.DOI:10.1016/S1003-6326(11)61343-5 |
