 ,1,2,3
,1,2,3Basicranial morphology of Late Miocene Dinocrocuta gigantea (Carnivora: Hyaenidae) from Fugu, Shaanxi
XIONG Wu-Yang ,1,2,3
,1,2,3通讯作者: xiongwuyang@ivpp.ac.cn
收稿日期:2019-04-1网络出版日期:2019-10-20
| 基金资助: |
Corresponding authors: xiongwuyang@ivpp.ac.cn
Received:2019-04-1Online:2019-10-20

摘要
巨鬣狗(Dinocrocuta gigantea)之前曾因其特殊的乳齿特征而被排除于鬣狗科之外,而归入单独的中鬣狗科(Percrocutidae)。对一产自陕西府谷的巨鬣狗头骨后部进行了扫描和内部结构重建,详细描述了颅基部内外形态,并与其他猫形类进行了对比。巨鬣狗在颅基部显示出了鬣狗科的典型模式,支持将其置于鬣狗科之内的传统观点,而中鬣狗科的有效性则值得怀疑。巨鬣狗的颅基部还具有很多独特特征,暗示其可能是鬣狗科的一个早期旁支。
关键词:
Abstract
Dinocrocuta gigantea was once moved out of the Hyaenidae based on its peculiar deciduous teeth and placed in a separate family - the Percrocutidae. A D. gigantea skull from Fugu, Shaanxi Province is scanned with the internal structures of its bulla reconstructed, described in detail, and compared with other feliforms. The basicranium of D. gigantea shows a typical hyaenid pattern, which supports the traditional view that it should remain within the Hyaenidae and questions the validity of the Percrocutidae. The basicranium of D. gigantea also possesses a number of unique features, suggesting that it could be an early side-branch of the Hyaenidae.
Keywords:
PDF (7349KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
熊武阳. 陕西府谷晚中新世巨鬣狗(Dinocrocuta gigantea)(食肉目:鬣狗科)颅基部形态研究. 古脊椎动物学报[J], 2019, 57(4): 274-307 DOI:10.19615/j.cnki.1000-3118.190710
XIONG Wu-Yang.
Percrocuta and Dinocrocuta were considered as early representatives of the modern durophagous hyena lineage for their extremely hypertrophied premolars and hypercarnivorous carnassials (Kurtén, 1956, 1957). Subsequent studies, however, found that these animals seem to have acquired hyaenoid dental adaptation since the Middle Miocene, and are more advanced than most of the other contemporary hyaenids (Thenius, 1966). Later investigations revealed the even more striking fact that the lower deciduous carnassials (dp4) of Percrocuta and Dinocrocuta are sharply different from those of other hyaenids and extant viverrids, but similar to those of the Oligocene Stenoplesictis and felids (Schmidt-Kittler, 1976; Chen and Schmidt-Kittler, 1983), which implies that Percrocuta and Dinocrocuta originate from Stenoplesictis close to felids and convergent to true hyaenids. Following this demonstration, Werdelin and Solounias (1991) erected a new family, the Percrocutidae, for these genera and closely related forms and excluded them from their study of hyaenid phylogeny.
Since the investigation of Schmidt-Kittler, numerous works on the basicranial anatomy of the feliforms have been made by Hunt (1987, 1989, 1991, 1998), Hunt and Solounias (1991), Hunt and Tedford (1993), resulting in a split of the classical paraphyletic Viverridae, which included all feliforms except felids and hyaenids, into the monophyletic Nandiniidae, Viverridae and Herpestidae, which could be defined by their unique basicranial patterns, and a new scheme of the feliform phylogeny was established where the systematic position of many fossil taxa like Stenoplesictis and Herpestides were relocated. Taking into consideration Hunt’s works and later molecular analyses on the carnivoran phylogeny (e.g., Flynn et al., 2005), Schmidt-Kittler’s supposition of the Percrocutidae based on the features of Stenoplesictis and the paraphyletic “viverrids” is now quite problematic.
Observation and interpretation of the basicranial features of the Percrocutidae are crucial with respect to its affinity as well as the phylogeny of Feliformia. While percrocutids are recorded in many localities in the Old World (Howell and Petter, 1985), well-preserved basicranial materials are only reported in Dinocrocuta gigantea materials from Hezheng and Fugu, northwestern China (Qiu et al. 1988a; Zhang and Xue, 1996). In a report of the Hezheng material, Qiu and coauthors briefly noticed a large mastoid process and an anteriorly concave bulla in the auditory region of D. gigantea. However, the information within the bulla remains unknown.
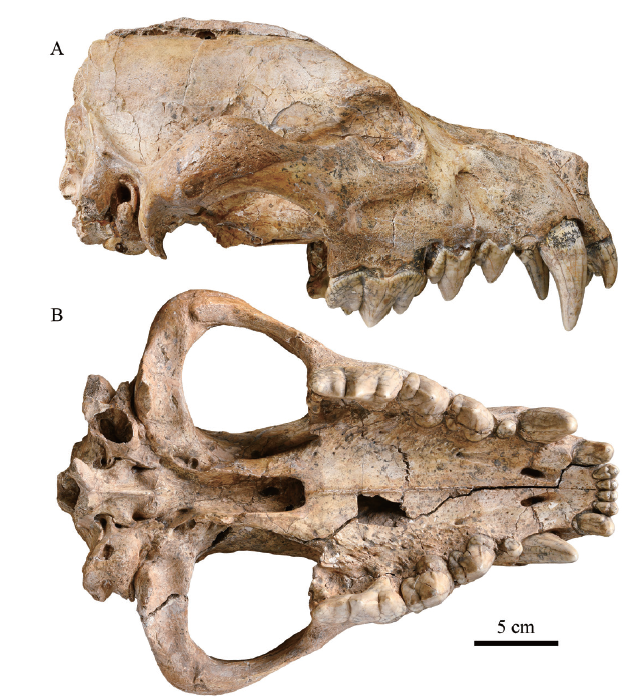
Here, IVPP (Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences) V 15649 (Fig. 1), a well-preserved D. gigantea skull from Fugu County, Shaanxi Province, China with some part of the infillings naturally removed is chosen for CT scanning and a detailed study on the basicranium.
Fig. 1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 1Photograph of Dinocrocuta gigantea (IVPP V 15649) in lateral (A) and ventral (B) views from Fugu County, Shaanxi Province
1 Materials and methods
The skull is broken into two parts with a long crack. Dorsally the crack runs roughly midsagittally. Ventrally the crack begins between right I2 and I3, cuts obliquely and ends in the left glenoid fossa, leaving the basipharyngeal canal and the basicranial region in the right half. The basal length of the skull is about 316 mm, and the zygomatic width is 248 mm.The upper dentition is completely preserved. All teeth are only slightly worn at the tips. The left canine is not fully erupted compared to the right one with its crown about 1 cm long unexposed. Many bone sutures are still well defined, depicting the nature of a young adult.
The posterior part of the sagittal crest, the surface of the supraoccipital, the occipital condyles and the paracondylar processes are broken off, and the bilateral nuchal crests and mastoid processes are only partially preserved. A considerable portion of the wall of the right bulla is lost with its chamber exposed. Bilateral external acoustic meatus are partially hollowed out. These facts imply that the posterior end of the skull was exposed in the open air for some time, and the infilling in the basicranial space may have been at least partially removed by the water.
The skull was scanned by Tseng (2009) for comparative finite element analysis. He noticed some of the internal cranial features such as the super-sized frontal sinus, but the resolution of this scanning didn’t allow for a detailed study.
In this study, the right part of the skull was scanned using the 450 kV micro-computerized tomography apparatus (developed by the Institute of High Energy Physics, Chinese Academy of Sciences (IHEP)) at the CAS Key Laboratory of Vertebrate Evolution and Human Origins. The specimen was scanned with a beam energy of 450 kV and a flux of 1.5 mA at a resolution of 160 μm per pixel using a 360° rotation with a step size of 0.25° and an unfiltered aluminum reflection target. A total of 1440 transmission images were reconstructed in a 2048×2048 matrix of 2048 slices using a two-dimensional reconstruction software developed by the IHEP.
The scanning image shows that the matrix of the left bulla is porous and poorly indurated. The boundary between the bony wall and the space of the left bulla is overall well-defined, and ideal for a reconstruction of the internal structures. Probably the soluble component of the infilling has been removed by water through the hollowed external acoustic meatus. The right bulla, despite being exposed, contains matrix, which is dense and tightly adhered to the bony walls and provides only complementary information for reconstruction.
The reconstruction is carried out using Mimics 18.0 (Materialise N.V., Leuven, Belgium).
In addition to the fossil material, six extant feliform skulls are scanned for comparison, including IVPP OV 232 (spotted hyena Crocuta crocuta), OV 1469 (striped hyena Hyaena hyaena), OV 40 (subadult tiger Panthera tigris), using the 450 kV micro-CT in a resolution of 160 μm, and OV 197 (aardwolf Proteles cristatus), OV 243 (white-tailed mongoose Ichneumia albicauda), OV 635 (juvenile masked palm civet Paguma larvata), using the 225 kV micro-CT in a resolution of 47, 37, 37 μm respectively. Several other Dinocrocuta gigantea skulls, including HMV (Hezheng Paleozoological Museum) C 0123, M 0358, X 2149, and IVPP RV 88002, all from the Late Miocene of Linxia Basin, Gansu Province, are observed in order to add details of the structures not preserved in V 15649. In all these materials, the external morphology of basicranium shows a good uniformity.
The terminology follows principally Wible and Spaulding (2013), supplemented by van der Klaauw (1931), Macphee (1981), Evans and de Lahunta (2013). Several new terms are created for the convenience of description and comparison. For the name of the osseous canal in the anteromedial corner of bulla, the musculotubal canal is chosen here instead of the auditory tube. The latter name, as well as the names Eustachian tube and tympanopharyngeal tube, could be applied to the tube in the soft tissue anatomy, which is bounded by the mucous membrane and connects the cavum tympani to the nasopharynx. The osseous musculotubal canal would house not only the auditory tube but also the muscle bundle of the tensor veli palatini and some small vessels. For the name of the ventrolateral process of exoccipital developed behind the bulla, paracondylar process is chosen instead of the commonly used paroccipital process to avoid confusion with the petrosal portion of the mastoid process which is sometimes referred as the paroccipital process of petrosal (Wible and Spaulding, 2013).
2 Description
2.1 Bones adjacent to the bulla
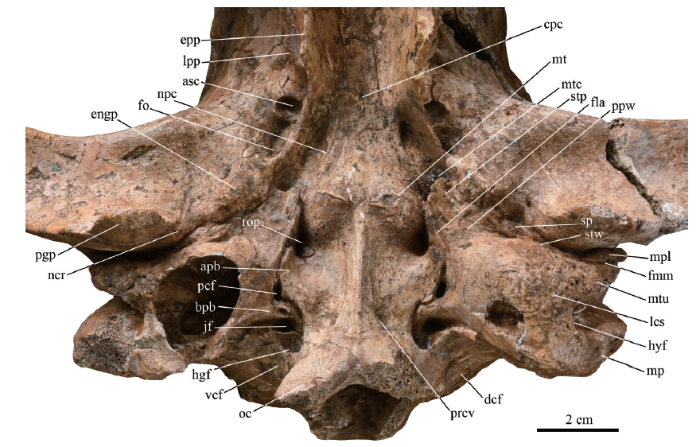
2.1.1 The pterygoid and the alisphenoidThe posterior portion of the basipharyngeal canal (also known as the nasopharygeal fossa) is covered by the pterygoids, except for at the middle part of the roof, which is formed by the presphenoid. The pterygoid fuses laterally with the alisphenoid forming the sidewall of the basicranial canal or the entopterygoid process (epp in Fig. 2). The palato-pterygoid suture on the medial surface of the entopterygoid process is somewhat more anteriorly positioned than the palato-alisphenoid suture on the lateral surface. The ventral margin of the process everts slightly in its posterior part. The hamuli are broken from their roots on both sides. The width between roots in V 15649 is 24.6 mm, almost equal to that in Proteles (OV 197: 22.9 mm), and smaller than in the extant Crocuta (OV 232: 30.4 mm), implying an extremely narrow nasopharynx relative to its broad snout.
Fig. 2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 2Photograph of the basicranium of Dinocrocuta gigantea (IVPP V 15649)
Abbreviations: apb. auriform process of basioccipital; asc. caudal opening of the alisphenoid canal;bpb. boot-like process of bulla; cpc. ventral opening of the craniopharyngeal canal; dcf. dorsal condyloid fossa; engp. entoglenoid protuberance; epp. entopterygoid process; fla. the “flat”; fmm. the fissure separating the meatal plate and the meatal tubercle; fo. foramen ovale; hgf. hypoglossal foramen; hyf. hyoid fossa;jf. jugular foramen; lcs. lateral part of bulla with the coarse surface; lpp. lateral pterygoid process; mp. mastoid process (broken); mpl. meatal plate; mt. muscular tubercle; mtc. musculotubal canal; mtu. meatal tubercle; ncr. notch for the chorda tympani nerve and the rostral tympanic artery; npc. groove for the nerve of pterygoid canal; oc. occipital condyle (broken); pcf. posterior carotid foramen; pgp. postglenoid process; ppw. the ventral ridge of the “perpendicular wall”; prcv. pit inserted by the rectus capitis ventralis; rop. the round pit ventral to the rostral entotympanic and the rostral tympanic process of petrosal; sp. spine of the tympanic plate of the mallear rostral process; stp. styliform process; stw. “steep wall”; vcf. ventral condyloid fossa
Lateral to the entopterygoid process is the weak lateral pterygoid process (lpp in Fig. 2), blunt rostrally and sharpened caudally to form an edge. At the caudal end of the lateral pterygoid process, a semi-columnar buttress stretches dorsocaudally and joins the lateral osseous wall of the alisphenoid canal perpendicularly. The latter encloses a large alisphenoid canal that merges with the canal for the foramen rotundum to produce a common rostral opening larger than the adjacent sphenorbital fissure. The lateral pterygoid process, the buttress and the lateral wall of the alisphenoid canal constitute the protrusion on the vertical portion of the alisphenoid.
Measured in the CT images, the alisphenoid canal is 5.5 mm in diameter. Its presence, substantiated in all accessible Dinocrocuta specimens where the corresponding region is preserved and prepared, is a primitive feature according to Werdelin and Solounias (1991). It’s noteworthy that the alisphenoid canal is found to be variable in extant hyaenids (Pocock, 1916b) as well as in the Yushe specimens of Adcrocuta eximia and Pliocrocuta perrieri (personal observation). According to Davis and Story (1943), Bugge (1978), the loss of an alisphenoid canal, i.e. the absence of the lateral osseous wall wrapping the internal maxillary artery which is the main branch of the external carotid artery, would leave more room for the development of an external rete mirabile. Hence, it’s reasonable to infer from the presence of a large alisphenoid canal in Dinocrocuta that the external rete mirabile in this animal would be less developed than those in extant felids and hyaenids.
Caudal to the hamulus, the entopterygoid process continues as the tympanic process of the alisphenoid, descends and laterally bends, dividing the ventral face of alisphenoid into two parts. Lateral to the process lies a depression that accommodates the caudal opening of the alisphenoid canal (asc in Fig. 2) in the front and the foramen ovale (fo in Fig. 2) in the rear. Medial to the process is a trough for the auditory tube that ends anteriorly with a long oval concavity. Ultimately, the entopterygoid process terminates at a strong protrusion that is termed the entoglenoid protuberance herein (engp in Fig. 2). There it joins the entoglenoid process of the squamosal (noted in Wible and Spaulding, 2013) as a ridge on the protuberance, the peak of which is situated where the ridge crosses the alispheno-squamosal suture and juts out about 8 mm below the plane of the glenoid fossa.
The entoglenoid protuberance, lying anterior to the bulla, medial to a notch for the chorda tympani nerve and the rostral tympanic artery (ncr in Fig. 2), and lateral to the opening of the musculotubal canal (mtc in Fig. 2), is unlikely to demarcate the temporomandibular joint capsule, as the notch for the chorda tympani nerve would thence lie within the capsule. A faint line running between the medial ridge of the postglenoid process and the notch for the chorda tympani nerve forward to the weak developed preglenoid process seems to limit the capsule medially. In the corresponding region, there’s only a small ridge with slight elevation or some wrinkles in other hyaenids, and no similar prominent protuberances could be found among other carnivorans.
2.1.2 The basisphenoid
The basisphenoid contacts with the presphenoid somewhat anterior to the pterygoid hamuli. Caudally, it abuts against the basioccipital at paired muscular tubercles at the same level with the anterior rim of the postglenoid process. Slightly anterior to the middle point of its central axis lies the ventral opening of the craniopharyngeal canal, which is cuneiform in shape (cpc in Fig. 2). In CT images, the craniopharyngeal canal runs anterodorsally to communicate the nasopharynx with the hypophyseal fossa.
The lateral borders of the basisphenoid diverge posteriorly. On each side, it is parallel to the entopterygoid process through their whole length, outlining a funnel shape typical of this bone. The posterior part of the lateral border is lined by a tiny groove for the nerve of pterygoid canal (npc in Fig. 2) representing the unenclosed portion of the pterygoid canal. The groove passes backward along the dorsomedial corner of the musculotubal canal.
2.1.3 The occipital
On each muscular tubercle (mt in Fig. 2), the basispheno-basioccipital suture arches forwards leaving most of the roughened surface for the attachment of longus capitis muscle on the basioccipital. In older individuals of Dinocrocuta, the tubercle is slightly anteriorly elongated. In Hyaena, Crocuta, Adcrocuta, and Pachycrocuta, the muscular tubercles are often flattened, laterally appressed to the bullae, and the basisphenoid surface in front of them are swollen forming a pair of variably developed parasagittal ridges which was also noticed by Qiu (1987) and Werdelin and Solounias (1991). In comparison, the tubercles in Dinocrocuta are more rounded in shape and more pronounced from the surface of the basisphenoid. Among other carnivorans, the muscular tubercles are rarely developed.
Laterally the basioccipital borders the bulla with some notable features. This border can be divided into three subequal parts. The rostral and caudal thirds remain parasagittal in general. The middle part juts out laterally, ventrally and somehow anteriorly producing an auriform process that reclines on the medial wall of the bulla (apb in Fig. 2). The auriform process has three sides. The anterior side and the rostral third of the border form a notch that faces anterolaterally and encloses a round pit with the medial wall of the bulla (rop in Fig. 2). The lateral side of the process is convex anteriorly and concave posteriorly, lying at an obtuse angle to the posterior side. The concave portion of the lateral side outlines together with a trough on the medial side of the bulla the posterior carotid foramen (pcf in Fig. 2, further discussed in 2.2.3).
The auriform process of basioccipital is also present in Crocuta and Pachycrocuta (in Hunt, 1974:fig. 37, and personal observation). In these hyaenids, the process is thin, smoothly passing onto the neighboring bulla surface, often easily overlooked, whereas in Dinocrocuta it is thicker and more outstanding from adjacent structures. The ventrally down turning lateral edge of the basioccipital is common in feliforms, further development of which into a flange is also recorded (Hunt, 1991). The auriform process could originate from a similar flange. The basioccipital flange is regarded as the muscular tubercle in Nandinia by Wible and Spaulding (2013), which is not the case at least in Dinocrocuta where the muscular tubercle is located on the basisphenoid-basioccipital suture.
The posterior side of the auriform process adjoins the boot-like process of bulla (bpb in Fig. 2) which expands medially at first and then curves caudally bordering the caudal third of the basioccipital. Posterolaterally, the boot-like process encloses the jugular foramen (jf in Fig. 2) with the jugular notch of the exoccipital. It is visible in Crocuta and Pachycrocuta as well. In Hyaena and Proteles, the boot-like process of bulla is less distinct, though a posteromedial protrusion is apparent.
Behind the jugular foramen lies a slightly larger hypoglossal foramen (hgf in Fig. 2), which is roughly round and opens caudally, dorsally, and a bit medially into the cranial cavity. Behind the jugular notch, two ridges encircle the hypoglossal foramen. One originates at the tip of the boot-like process of bulla and runs straight laterally and ventrally as a partition between the jugular foramen and the hypoglossal foramen. The other is a continuation of the lateral border of the basioccipital that curves anterolaterally. Both ridges sharpen ventrally and converge laterally forming the ventral margin of the paracondylar process. The former ridge is lower than the latter, yet it is farfetched to say that the jugular foramen and the hypoglossal foramen are situated in a common fossa, a condition commonly known in modern hyaenids and felids. These two foramina are separated, but not to the degree that they are in the plesiomorphic condition of Nandinia, Prionodon, nimravids as well as that of canids and arctoids (Hunt, 1987, 2001). A similar condition is noted in the gigantic lion Panthera leo atrox by Hough (1953), so this state could be interpreted as being either plesiomorphic or relevant to allometry and gigantism.
Along the midsagittal line of the basioccipital, a keel originates between the muscular tubercles and ends where the paired occipital condyles approach each other ventrally. While extending caudally, the keel increases in both breadth and height, thus forming a funnel-shaped elevation in the ventral aspect, as well as an angle of about 8° to the basicranial plane. On either side of the keel lies a faint pit near the condyle where the rectus capitis ventralis muscle is presumably inserted (prcv in Fig. 2). In respect to the ventral contiguity of the occipital condyles noted by Buckland-Wright (1969), Dinocrocuta resembles Hyaena, rather than Crocuta.
Most parts of the occipital condyles (oc in Fig. 2), the entire dorsal rim of the foramen magnum except a faint dorsalmost notch are broken away. The dorsal portion of the occipital surface, the left nuchal crest, and the bilateral distal parts of the paracondylar process are also not preserved. These structures are briefly described and photographed by Qiu et al. (1988a), Zhang and Xue (1996). The occipital condyles are somewhat ventrally shifted, which is associated with the ventrally angled midsagittal keel described above. The upper part of the condyle is laterally rotated. Correspondingly, the ridge dividing the dorsal and the ventral condyloid fossa (dcf and vcf in Fig. 2) is inferiorly positioned, making the latter smaller than the former. A similar angulation is observed to a lesser extent in Crocuta and Pachycrocuta, which could be interpreted to be an adaptation for supporting a larger head with a more erected neck.
The paracondylar process of Dinocrocuta has been figured by Qiu et al. (1988a) and Zhang and Xue (1996). It’s intact in HMV C 0123. Based on these materials, the description of its morphology is added here. In the posterior view, the outline of the paracondylar process is rounded; its lateral margin is gently curved with its lateralmost point at the same height with the upper end of the condyle. The proximal part of process covers the posterior face of the bulla. The distal part is a thin triangular plate that projects posteriorly and ventrolaterally. In the ventral view, the base line of the triangular plate is its suture with the bulla, the vertex of the triangle is more than 1 cm away from the base line and slightly roughened for the digastricus muscle. In other hyaenids, the lateral edge of the paracondylar process is straight and nearly vertical, along which a ridge is often developed. In large felids and most large hyaenids, the tip of this process is a nodule that bends anteriorly from the posteroventral corner of the bulla in contrast to the triangular plate in Dinocrocuta that turns posteriorly without apparent thickening.
Under CT investigation, the inferior petrosal sinus sulcus of Dinocrocuta is found to be wide and shallow, lying on the dorsal surface of the basioccipital without contacting the petrosal (Fig. 3A). In Crocuta (Fig. 3B) and Proteles, its position and shape are almost the same. However, in Panthera (Fig. 3D, also depicted in Flower, 1869:fig. 7) and Paguma (Fig. 3E), the petrosal forms the lateral wall of the sulcus, which is identical to the case in Nandinia (Wible and Spaulding, 2013). In Panthera, a medial crest of the petrosal roofs a portion of the sulcus, and the caudal entotympanic floors it ventrolaterally. In Ichneumia (Fig. 3C), the inferior petrosal sinus is enclosed by the basioccipital from the medial side and the petrosal from the lateral side forming a petrooccipital canal similar to that of dogs (Evans and de Lahunta, 2013). In general, the position of the inferior petrosal sinus varies among different feliform groups and is likely to be an important feature in feliform phylogeny.
Fig. 3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 3Coronal section of feliform basicrania across cochlea
A. Dinocrocuta gigantea IVPP V 15649; B. Crocuta crocuta OV 232; C. Ichneumia albicauda OV 243;D. subadult Panthera tigris OV 40; E. juvenile Paguma larvata OV 635
Arrows show the locations of inferior petrosal sinus sulcus. Scale bars = 1 cm
The thickness of the basioccipital in Dinocrocuta is 7-9 mm except at the keeled middle part, which surpasses 12 mm posteriorly. Comparatively, the basioccipital is only 2-4 mm thick in Hyaena and Crocuta, and slightly more than 1 mm thick in Proteles. A similar distinction in thickness is reported between Ursus spelaeus and U. arctos (García et al., 2007). As a result, the thickened basioccipital of Dinocrocuta laterally wraps the ventromedial margin of the petrosal (its rostral tympanic process, see 2.2.3 and 2.3.2) to bound the medial wall of bulla posterior to the round pit (Fig. 3A).
The posterior part of foramen magnum is prepared, and the remaining internal surface of the exoccipital is clearly defined in CT imaging for observation. In this region, the condyloid canal is completely absent. I examined this canal, which is present in all hyaenids, herpestids, and viverrids, in all extant feliform specimens in the IVPP collection. In 81 specimens of 20 felid species, the canal is absent unilaterally in three specimens, and bilaterally only in a Lynx lynx (IVPP OV 2137). The venous drainage of the basilar sinus, which is housed in the condyloid canal, may be compensated in Dinocrocuta by the vein in the hypoglossal canal, as the latter canal is sizable according to the diameter of the hypoglossal foramen.
2.2 External morphology of the bulla
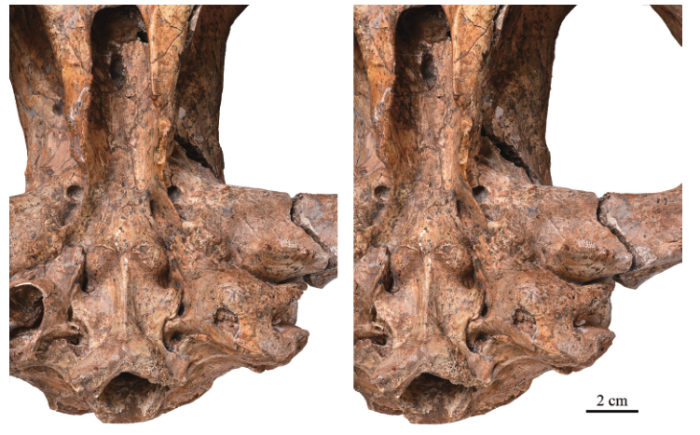
2.2.1 General external morphologyThe left bulla is nearly intact with only a small piece of the bony wall crushed inwards (Fig. 4), thus a detailed description is allowed for. In ventral view, the absolute size of this bulla is comparable to that of Crocuta crocuta. Proportionally, the bullae and the basioccipital are less anterior-posteriorly expanded than that of the extant hyaenids.The globular bulla bulges downwards strongly, with its lowest point about 16 mm ventral to the basicranial plane, almost equal to the tip of the postglenoid process. It gently slopes to bound the basioccipital medially, external acoustic meatus laterally and the mastoid process posterolaterally. The posterior aspect of the bulla, lying perpendicular to the basicranial plane, is originally covered by the paracondylar process and exposed bilaterally in the specimen.
Fig. 4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 4Photograph of the left auditory region of Dinocrocuta gigantea (IVPP V 15649), stereopairs
2.2.2 The anteromedial region of the bulla
The forepart of the bulla shows its most peculiar aspect. Slightly anterior to the rear surface of the postglenoid process, the bulla wall descends abruptly forming a “steep wall” (stw in Fig. 2) that stretches transversely. In front of the “steep wall”, the bulla wall is flat, and lies roughly in the basicranial plane, forming a “flat” (fla in Fig. 2) that ends anteromedially with the styliform process (stp in Fig. 2) as the floor of the musculotubal canal. Owing to the process and “flat”, the opening of the musculotubal canal in Dinocrocuta is located medial to the entoglenoid protuberance and the glenoid fossa, more anterior than in other carnivorans.
The styliform process, a delicate fan-shaped bony plate, is about 6 mm long and 7 mm wide. In the left bulla, most of its distal margin except at the medial end is retained, and the process curls from the margin laterally and ventrally. Among carnivorans, this process receives most of its attention in nimravids (Hunt, 1987; Joeckel et al. 2002). The styliform process in nimravids is protuberant, internally concave, and presses the alisphenoid at its base facing anterolaterally, thus it differs from that of Dinocrocuta, which faces posterolaterally with a slightly concave external surface. In ictitheres and extant Hyaena, the process can hardly be recognized, whilst in Adcrocuta, Pachycrocuta, extant Crocuta, Proteles, and medium-sized felids the process is quite wide, occupying most of the width of the musculotubal canal, but only 2-3 mm in anteroposterior length. The process described here bears the most resemblance to those of large Panthera, which have a similar size, shape, and orientation. It seems that the development of the styliform process is relevant to both size and phylogenetic relationship.
The lateral margin of the styliform process in Panthera and Crocuta curves postero-dorsally into the musculotubal canal as a faint ridge running along the ventrolateral edge of the canal, marking the boundary between the dorsolateral moiety of the canal for the muscle bundle of the tensor veli palatini and the ventromedial moiety for the auditory tube. In some canids and ursids, this ridge is a prominent partition dividing the canal into two semicanals (Davis, 1964; Evans and de Lahunta, 2013). In these aforementioned carnivorans, there is a notch at the lateral limit of the musculotubal canal between the lateral edge of the styliform process and the processus tubarius, which is located lateral to the canal and projects forward from the bulla (van der Klaauw, 1931). This notch, representing the ventrolateral range of the tensor veli palatini, doesn’t occur in Dinocrocuta. In other words, the styliform process in Dinocrocuta touches the processus tubarius laterally, inferring that the tensor veli palatini bundle doesn’t occupy the ventrolateral limit of the musculotubal canal at least at the bony opening, and situates more dorsally to the auditory tube. The processus tubarius of Dinocrocuta is poorly developed and pressed from the front by the entoglenoid protuberance.
The styliform process is reported to supply an insertion of the levator veli palatini (Evans and de Lahunta, 2013). A shallow depression at the base of the process, misidentified in cats as the scaphoid fossa by Stowell (1888), may indicate the exact location of the muscle attachment, so it seems that the “flat” behind the process in Dinocrocuta may augment such an attachment. In those taxa where the styliform process is poorly developed, the origin of levator veli palatini would shift further forwards at the ventral aspect of the proximal non-osseous tube, as is figured by von Kostanecki (1891) in Phoca vitulina. Cave (1979) noted a temporo-pterygoid ligament that derives from a caudal thickening of the tensor veli palatini and connects the pterygoid hamulus with an “Eustachian process” of the bulla. His Eustachian process is located either lateral or medial to the auditory tube among different taxa, thus it is probably not homologous. Moreover, the auditory tube that he located in the herpestid Bdeogale is indeed occupied by the internal carotid artery. Given the fact that he didn’t include a carnivoran in the detailed anatomy of soft tissue, the relationship between the temporo-pterygoid ligament and the styliform process is herein difficult to speculate on further.
The anterior portion of the medial bulla wall lies perpendicular to the basicranial plane. The ventral margin of this “perpendicular wall” forms a sharp ridge (ppw in Fig. 2) that stretches between the styliform process and the muscular tubercle posterolaterally to the distal margin of the auriform process of the basioccipital, separating the “flat” and the basioccipital. The “perpendicular wall” and its sharp ridge characterize the anterior bulla region of Dinocrocuta. In extant hyaenids and large felids, the configuration of this region is totally different. The long anteromedial process, flat or linguiform in felids and more conical or literally styliform in hyaenids, sticks out forward medial to the opening of the musculotubal canal and the groove for the pterygoid canal, and sometimes surpasses the rear edge of foramen ovale. According to the juvenile feliform basicrania figured by Hunt (1987, 1989), it can be assumed that the anteromedial process is a part of the rostral entotympanic. It is short in all juvenile feliforms and elongates anteriorly during puberty on the surface of the basisphenoid in hyaenids and large felids, then gradually merges with the adjacent bones in older adults. In canids and arctoids, this process is perforated or bifurcated due to the perbullar course of the internal carotid artery, whereas in other taxa mentioned by van der Klaauw, 1931 and Macphee, 1981 the homonymous anteromedial process may not be homologous to the process in carnivorans as a rostral entotympanic is not affirmed in some of these taxa. Therefore, this process is more properly called the anteromedial process of rostral tympanic in carnivorans. In Dinocrocuta, this process is completely absent, with part of its position occupied by the aforementioned “perpendicular wall” and no further elongation medial to the musculotubal canal. Under CT observation, the “perpendicular wall” doesn’t show a histological continuity with the supposed rostral entotympanic.
2.2.3 The medial region of the bulla
Medial to the “perpendicular wall” and anterior to the auriform process occurs the round pit. Under the CT observation, this pit penetrates dorsally in a depth of 8 mm from the basicranial plane and gradually diminishes in size, to meet the anterior slope of the rostral tympanic process of petrosal and the rostral entotympanic lying on the slope. These structures are further discussed in the description of internal structures on the roof of the anterior chamber below (2.3.2).
Posterior to the pit lies another foramen, which is about 5 mm long and olivary in shape on the ventral surface, bounded medially by the concave margin of the auriform process, posteriorly by the boot-like process, and laterally by a trough on the bulla wall. This foramen is considered as the posterior carotid foramen for two reasons:
(1) Under CT observation, the foramen can be traced to the rear part of the rostral tympanic process of petrosal and further forwards, implying a transpromontorial course of the internal carotid artery typical of feliforms (Hunt, 1989).
(2) In Crocuta, the posterior carotid foramen is located posterolateral to the auriform process, and in other feliforms the foramen is often closely related to the posterior aspect of the basioccipital flange as well (e.g. in Arctictis, Hunt, 1987). A medial buttress of bulla behind the posterior carotid foramen is common in non-felid feliforms. In Crocuta, the buttress curves somewhat posteriorly; thus, it shows an incipient transformation to the boot-like process of bulla in Dinocrocuta.
De Beaumont (1969) reported in Plioviverrops a bulla similar to that of Dinocrocuta in bearing two subequal foramina along its medial margin. He labeled one as posterior carotid foramen and related the other to the inferior petrosal sinus, which could not be affirmed in any feliforms observed here.
2.2.4 The anterior region of the bulla
The anterior rim of the right bulla slightly juts forward near the notch for the chorda tympani posterolateral to the entoglenoid protuberance, while on the left side the jut can hardly be recognized (sp in Fig. 2). Wible and Spaulding (2012) assume that this jut is the spine of the tympanic plate of the mallear rostral process that fuses with the ectotympanic as a part of the bulla in the adult. A similar spine can be found in Crocuta that is blunt, flattened on the surface of squamosal, and differs from those in felids, viverrids, and herpestids that are anteriorly pointed and sometimes elongated. In Proteles, the spine is a flat triangular crest bearing a free end about 3 mm apart from the squamosal surface.
Posterior to the spine, the “flat” in front of the “steep wall” diminishes laterally and the wall becomes more vertical. At some point, the “corner” of the “steep wall” is even recessed posterodorsally, depicting a sigmoid contour in the parasagittal section of bulla. Further laterally, the transverse steep wall bends a little posteriorly in transit to the external acoustic meatus.
In Dinocrocuta, the space between the “steep wall”, the postglenoid process and the anterior aspect of the external acoustic meatus (named as a curvilinear recess by Buckland-Wright (1969) in extant hyenas) becomes a fissure significantly narrower than in any other carnivorans (clr in Fig. 5A). This results from the strong backward shift of the glenoid fossa. The postglenoid process lies posterior even to the basispheno-basioccipital suture approaching the bulla and the meatus. Such a shift is observed to a lesser extent in canids, the giant panda and other durophagous hyaenids, and is probably related to the strong bite. Within the curvilinear recess, the postglenoid foramen is absent, which is typical in Feliformia where the temporal sinus is commonly reduced.
Fig. 5
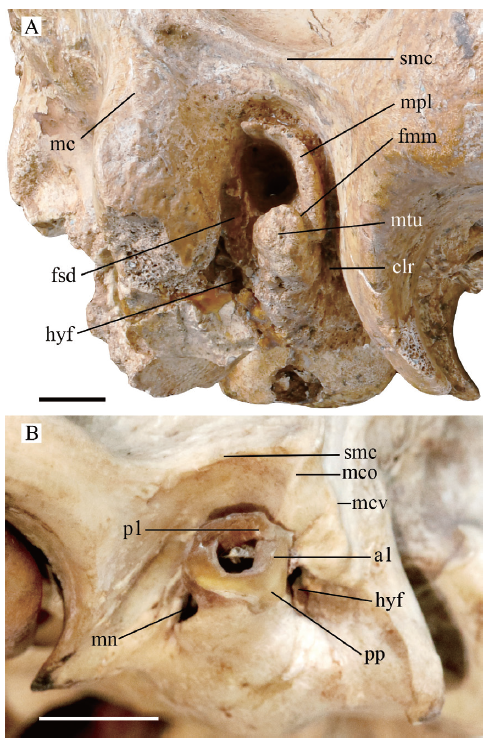 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 5Photograph of the lateral osseous external acoustic meatus and the mastoid process
A. Dinocrocuta gigantea IVPP V 15649 (right side);B. Canis familiaris OV 497 (left side)
Abbreviations: a1. anteron 1 of the annular cartilage;clr. curvilinear recess; fsd. foramen stylomastoideum definitivum; mc. mastoid crest (vertical branch); mco. the oblique branch of the mastoid crest; mcv. the vertical branch of the mastoid crest; mn. meatal notch;p1. posteron 1 of the annular cartilage; pp. proximal process of the annular cartilage; smc. suprameatal crest
Other abbreviations see
2.2.5 The lateral osseous external acoustic meatus
The external acoustic meatus is distally 11 mm in vertical diameter and 8 mm in horizontal diameter. Its space is overall cylindrical associated with tubular osseous structures that protrude medially and laterally from the lateral wall of the bulla (Fig. 6), which are termed here as the medial and lateral osseous external acoustic meatus respectively to avoid ambiguity. The lateral osseous external acoustic meatus includes two parts: a semicircular plate that wraps the meatus dorsally and anteriorly (mpl in Figs. 2, 5A) and a meatal tubercle that wraps the meatus ventrally (mtu in Figs. 2, 5A, 6). The former, previously mentioned by Buckland-Wright (1969) as the tympanic plate, is more appropriately called a meatal plate to avoid confusion with other structures such as the tympanic plate of the mallear rostral process. In Dinocrocuta, the lateralmost point of the meatal plate is at the ventral part of its anterior portion where it is as high as the salient suprameatal crest (smc in Fig. 5A) dorsal to it. The meatal plate vanishes at the posterodorsal corner of the meatus, and the hinder aspect of the meatus is devoid of lateral osseous protrusion so that the lateral osseous external acoustic meatus doesn’t form a perfect tube. The meatal wall medial to the meatal plate is slightly elevated from the wall of the hinder part, forming a tiny stair along the meatus.
Fig. 6
 新窗口打开|下载原图ZIP|生成PPT
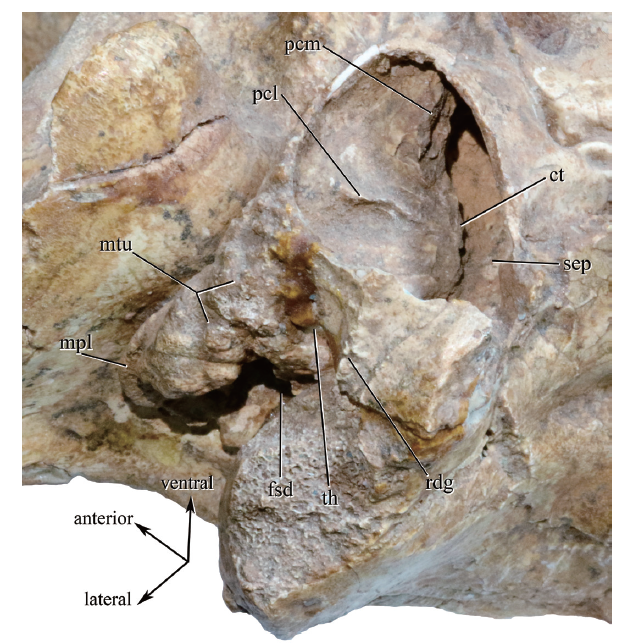
新窗口打开|下载原图ZIP|生成PPTFig. 6Photograph of the right bulla of Dinocrocuta gigantea (IVPP V 15649) in oblique ventral view showing structures around the medial and lateral osseous external acoustic meatus
Abbreviations: ct. crista tympanica; pcl. lateral parameatal crest; pcm. medial parameatal crest;rdg. the ridge raised along the caudal end of the borderline between the lateral coarse part and the medial smooth part on the surface of bulla; sep. anterior margin of the septum that abuts the promontorium in front; th. exit for the tympanohyal in the hyoid fossa. Other abbreviations see Figs. 2, 5. Not scaled
At the anteroventral corner of the meatus, a fissure runs medially along the meatus separating the meatal plate from the meatal tubercle (fmm in Figs. 2, 5A). Medial to the base of the meatal tubercle, this fissure bends somewhat anteriorly along the “steep wall” of the bulla and gradually diminishes. The tubercle equals the meatus in size distally, and becomes much larger proximally. In the shape of a tetrahedron, the meatal tubercle bears a dorsal face concave for the meatus, an anterior face contiguous with the “steep wall” and a posterior face, which is the largest, ventrolaterally slanting and rugose in its dorsal portion.
Comparatively, the lateral osseous external acoustic meatus is poorly develop-ed in Nandinia, felids, viverrids as well as the primitive hyaenids Protictitherium (Fraile, 2015), and the peculiar Tongxinictis (Qiu et al., 1988b and personal observation). Among these taxa and canids, a meatal notch is present at the anteroventral corner of the meatus, presumably homologous with the fissure of Dinocrocuta located in the same place. It probably acts as a loophole in the junction between the osseous meatus and the annular cartilage that corresponds to the entrance of a branch of the deep auricular artery into the middle ear cavity as is indicated in the work of Davis and Story (1943) on cats. In the ictithere and Adcrocuta specimens, the anteroventral notch persists, the ventral thickening is wanting, but the anterior portion of a meatal plate develops moderately. In Proteles, the plate is a well-developed semicircle that wraps the anterior half of the meatus, and the notch is missing. In Chasmaporthetes, the plate almost encircles the meatus forming a perfect tube without ventral thickening. In Pachycrocuta and Hyaena, the meatal plate is developed anteriorly and dorsally, and a small meatal tubercle appears in the ventral edge, whereas the meatal notch is absent. In Crocuta, the meatal plate is slightly shorter and thinner than the former two genera, and the meatal tubercle is weaker. In OV 232, a shallow meatal notch is present and more posteriorly located than that of canids, felids, ictitheres and Adcrocuta. Similarly, the notch of herpestids is located at the bottom of the meatus dividing an anterior meatal plate and a posteroventral one. The location of the notch is probably related to the direction of the ectotympanic crura and the gap between them in the juvenile, which is anteromedial in felids, viverrids and medial in herpestids according to the figures of Hunt (1987, 1989), assuming the meatal notch is a relic of the crura gap. The early development of ectotympanic in hyaenids is still poorly known. In the juvenile Crocuta described by Hunt (1974:fig. 35), the meatal notch is exactly in the same place as that of OV 232, and more developed.
In the Canis familiaris specimen OV 497, the annular cartilage is attached to the slightly developed lateral osseous external acoustic meatus (Fig. 5B). According to the description of Freund (1910), the cartilage is considered in situ here. At the anteroventral edge of the meatus, a meatal notch is present and free of contact with the annular cartilage (mn in Fig. 5B). Using the terminology of auricular cartilage proposed by Boas (1912), the medial edge of the cartilage encloses the meatus from the anteron 1 (a1 in Fig. 5B) clockwise to the posteron 1 (p1 in Fig. 5B) with these two ends meeting in the posterior aspect of the meatus. Adjacent to the anteron 1, the proximal process of the cartilage (pp in Fig. 5B) covers the vestigial osseous meatus at its posteroventral edge, which is comparable to the posterior surface of the meatal tubercle in Dinocrocuta. It’s likely that the annular cartilage of Dinocrocuta may have evolved a sturdy proximal process similar to the one described by Boas in Hyaena that attaches on the meatal tubercle.
2.2.6 The lateral region of the bulla
The mastoid process (mp in Fig. 2) is situated directly behind the lateral osseous external acoustic meatus. Between it and the meatal tubercle lie two foramina. One, lying at the posteroventral corner of the meatus, is the foramen stylomastoideum definitivum (fsd in Figs. 5A, 6), which opens laterally as the exit of the main trunk of the facial nerve, the auricular branch of vagus nerve, and the stylomastoid artery. The anterior surface of the mastoid process near this foramen is shallowly grooved for these vessels. The other, ventral to the foramen stylomastoideum definitivum, is the exit for the tympanohyal (th in Fig. 6). The bulla wall around this foramen is invaginated forming a hyoid fossa that faces ventrolaterally (hyf in Figs. 2, 5A). According to Takada et al. (2009), the hyoid fossa is attached by a tympano-styloid synchondrosis, which is often misidentified as the tympanohyal. The distance between these two foramina is about 6 mm, significantly larger than in other feliforms. In Hyaena and Crocuta, it is no longer than 2 mm. In Proteles, there’s only one foramen, meaning the two canals converge before their exits. The juxtaposition of foramen stylomastoideum definitivum and hyoid fossa in the Felidae is described by Salles (1992) as his character 26, where the foramen stylomastoideum definitivum is more separated in larger species. Despite adjoining the mastoid process, these two foramina in Dinocrocuta are different from those in large Panthera, machairodonts, and ursids, the latter being suppressed by the process to move medially and open downwards.
In the aspect of texture, the surface of the bulla can be divided into a lateral coarse part (lcs in Fig. 2) and a medial smooth part. The borderline between them begins near the entoglenoid protuberance, bends ventrally along the “steep wall”, draws posterolaterally and ends just behind the hyoid fossa where it is raised into a small ridge (rdg in Fig. 6). A similar textural difference is previously known among carnivorans in Barbourofelis (Baskin, 1981). Baskin regarded it as the boundary between ectotympanic and entotympanics, which is later rejected by Neff (1983) who mentioned the disaccord of the borderline location with the usual arrangement of the ectotympanic. Despite the fact that the borderline in Dinocrocuta, depicting a somewhat typical lateral semicircle, is not as peculiar as that in Barbourofelis where the musculotubal canal is linked to the posteromedial corner of the bulla, no histological or internal structural evidence could be found under CT investigation in support of it as a boundary of bullar components. Turnbull (1970) noticed in cats that a tendinous common raphe of the medial pterygoid muscle and superficial masseter is inserted on the lateral part of the bulla as well as the ventral aspect of the external acoustic meatus which could be interpreted as a posterior continuation of the insertion of these two muscles from the angular process of the mandible. Evans and de Lahunta (2013) mentioned the same raphe in dogs. Though they didn’t describe it in detail, it is clearly illustrated in their fig. 6-11 that the stylomandibular ligament, which connects the hyoid fossa to the angular process, represents the hindermost line of the raphe. Gill and Grant (1966) also noted the same relationship between the stylomandibular ligament and the medial pterygoid muscle in cats. Therefore, it’s reasonable to assume that the lateral rugosity of the Dinocrocuta bulla that ends at the hyoid fossa could be developed for an intensive attachment of this raphe. The backward approach of the temporomandibular joint to the lateral part of the bulla is probably associated with such an attachment.
Along the anterolateral corner of the bulla medial to the meatus, a depression is commonly present in Adcrocuta, Pachycrocuta, extant hyaenids, and other investigated feliforms. The corresponding part in Dinocrocuta is the “steep wall” and the strong bulging behind it without obvious depression, similar to those in Tongxinictis, Protictitherium, and ictitheres.
2.2.7 The mastoid process
The mastoid process is massively built, about 18 mm long measured from the hyoid fossa to its lateral end. In the side view, a mastoid crest branches off the nuchal-suprameatal crest, runs down and ends below the meatal tubercle (mc in Fig. 5A). When intact, the process comprises three facets, a smooth posterior one, continuous with the posterior bulla wall and the exoccipital, and the remaining two which are roughened, pitted, and separated from the former by the mastoid crest, including a larger one facing anteriorly and laterally as well as a small one facing ventromedially. In other carnivorans with a developed mastoid process, the mastoid crest often bifurcates into a vertical branch which is identical with the crest in Dinocrocuta and an oblique branch that draws forwards (mcv and mco in Fig. 5B). Based on the sutures in juvenile and subadult specimens, it is inferred that the oblique branch runs on the squamosal portion of mastoid, i.e. the posttympanic process of squamosal, and the vertical branch runs on the petrosal portion, the paroccipital process of the petrosal. It is noteworthy that the mastoid ridge should not be confused with the “paramastoid crest” mentioned by Buckland-Wright (1969), which is the lateral rim of the exoccipital.
In extant and most fossil hyaenids, it’s likely that the petrosal portion of mastoid has fused laterally with the encroaching caudal entotympanic, which enwraps the posterior chamber of the bulla (further discussed in the internal morphology of posterior chamber below), so that the vertical branch of mastoid crest virtually runs on the lateral wall of the posterior chamber. The oblique branch of hyaenids is weak and short, inferring a poorly developed posttympanic process of the squamosal. Given its absence in Dinocrocuta, a squamosal contribution to the mastoid process may not exist in the animal at all. Other than Dinocrocuta, the only known hyaenid possessing a protruding mastoid process independent from the bulla is Chasmaporthetes. In the Longdan Chasmaporthetes progressus specimen HMV 1196, the lateral end of the mastoid process bends posteriorly (Qiu et al., 2004:Pl. XII-1b, and personal observation). The mastoid processes of Dinocrocuta and Chasmaporthetes probably evolved independently and whether the latter is free of invasion from the posterior chamber is still unknown.
Compared with typical hyaenids having their muscles that normally originate on the mastoid process attached to the de facto bulla wall (Buckland-Wright, 1969; Spoor and Badoux, 1986), Dinocrocuta and Chasmaporthetes would have a larger facet facing anterolaterally in front of the vertical mastoid crest on the process, offering a larger area for the attachment of the sternomastoideus muscle.
On the fracture surface, the process of Dinocrocuta shows its cancellous nature, which is consistent with the CT observation.
2.3 Internal morphology of the bulla
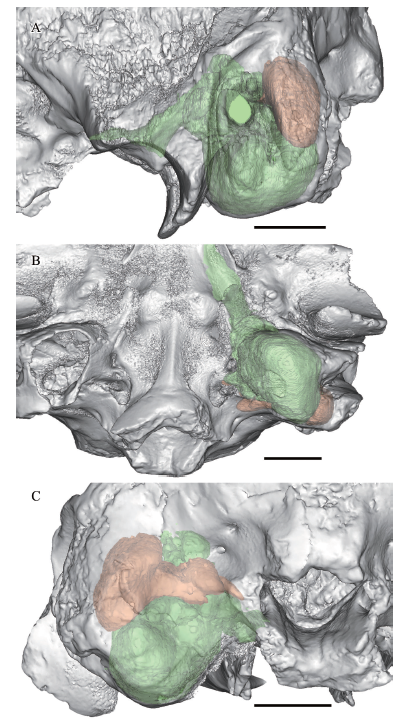
The bulla is an osseous complex that contains two chambers, typical of a feliform. These chambers are almost completely wrapped around by the bony wall of the bulla, except that a portion of their roof is exposed to the promontorium, tegmen tympani, and squamosal. The epitympanic wing of the alisphenoid forms the roof of the osseous musculotubal canal. In CT observation, the osseous complex is distinct from neighboring bones with the sutures, except along the osseous external acoustic meatus where it is merged with the squamosal, and in the mastoid process where it is merged with both the squamosal and petrosal.A large anterior chamber occupies the entire ventral vicinity of the bulla, while the posterior chamber is pushed to the remaining posterodorsal corner (Figs. 7, 8). No suture remains as direct evidence for the configuration of the bullar components in Dinocrocuta. Hunt (1987, 1989, 1991) shows that in most, if not all, advanced feliforms the anterior chamber is developed mainly from the ectotympanic, the posterior chamber is developed from the caudal entotympanic, and the septum is formed by both the ectotympanic and caudal entotympanic. While in the studies of Hunt the presence of this pattern in Hyaenidae was relatively less demonstrated than in other major feliform groups, and Ivanoff (2001) reviewed other hypotheses on the development of the hyaenid bulla, it’s generally adopted that the bulla walls of the anterior chamber and the posterior chamber would indicate the overall ranges of the ectotympanic and the caudal entotympanic respectively.
Fig. 7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 7Partial cranium of Dinocrocuta gigantea (IVPP V 15649) with reconstructed left bulla chambers semitransparently shown in lateral (A), ventral (B), and posterior (C) views
The anterior chamber is in green, the posterior chamber is in orange. Scale bars = 2 cm
Fig. 8
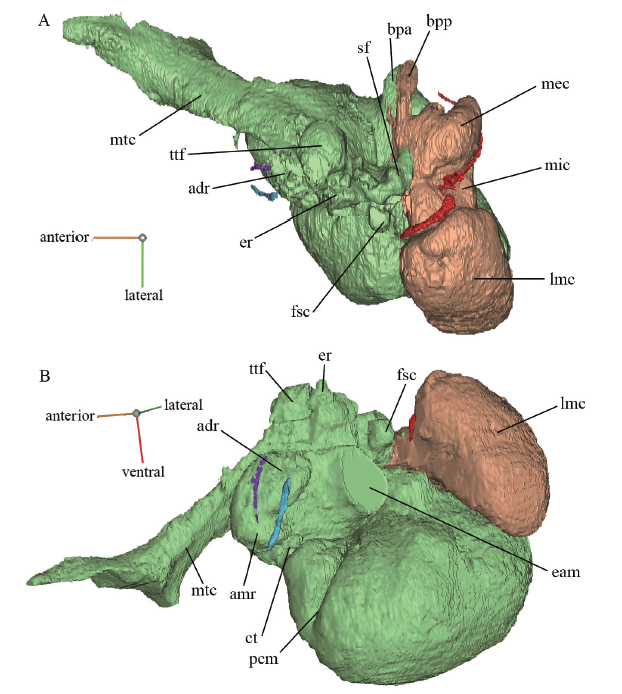 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 8Reconstructed internal structures in the left bulla of Dinocrocuta gigantea (IVPP V 15649) in dorsal (A) and anterolateral (B) views
The anterior chamber is in light green; the posterior chamber is in orange; the presumed hiatus for the lesser petrosal nerve is in purple; the canal for the rostral tympanic artery is in cyan; the mastoid canaliculus is in red
Abbreviations: adr. the small recess anterodorsal to the tympanic membrane; amr. the anteromedial recess dorsal to the “flat”; bpa. invasion of the boot-like process of bulla from the anterior chamber; bpp. invasion of the boot-like process of bulla from the posterior chamber; eam. space of external acoustic meatus (truncated); er. epitympanic recess; fsc. the rounded fossa presumably inserted by Spence’s cartilage; lmc. lateral mastoid cavity of the posterior chamber; mec. medial cavity of the posterior chamber; mic. middle constriction of the posterior chamber; mtc. musculotubal canal; sf. stapedius fossa; ttf. tensor tympani fossa
Other abbreviations see
2.3.1 The anterior chamber
The internal contour of the anterior chamber corresponds well with the external shape of the bulla. The main part of the anterior chamber is the hypotympanic sinus enwrapped by the bulging part of the bulla behind the “steep wall”. Anteromedially the floor of the chamber is terraced because of the “steep wall” and the “flat”. Dorsal to the “flat” the chamber is continuous into a deep anteromedial recess adjacent to the proximal end of the musculotubal canal at the middle ear proper (amr in Fig. 8). The angulation of the musculotubal canal is about 30° from the sagittal plane and about 45° from the basicranial plane. Posteromedially the anterior chamber invades into the boot-like process of the bulla, and posterolaterally it approaches the root of the mastoid process.
The medial osseous external acoustic meatus The most conspicuous structure inside the anterior chamber is the medial osseous external acoustic meatus (Fig. 6) which is a short tube enclosing the proximal meatus. The tube has an internal wall facing the meatal space and an “external” or centrifugal wall facing the space of the anterior chamber. Its free end sharpens and forms the crista tympanica outlining the elliptical pars tensa of tympanic membrane (ct in Figs. 6, 9). The anteroventral edge of crista tympanica extends much farther medially than the dorsal edge (ct in Fig. 8), being about 24 mm medial to the lateral wall and 8 mm to the dorsal edge, so that the tympanic membrane faces the chamber medially, dorsally and posteriorly. With the aid of location and measurement in CT imaging by Mimics software, the long axis of the elliptical tympanic membrane is estimated to be 15 mm long, and the plane of the tympanic membrane forms an angle of 63° with the horizontal basicranial plane and 30° with the sagittal plane. At the posterodorsal edge, a section of the meatal rim, in lack of the crista tympanica, presumably represents the “Tympanicumdefekt” of Bondy (1907) that connected with the Shrapnell’s membrane or pars flaccida of tympanic membrane. A posterior tympanic spine is present at the posterior junction between the crista tympanica and Tympanicumdefekt.
On the internal wall of the medial osseous external acoustic meatus, there is no apparent concavity. At the anteroventral edge of the meatus, the surface of wall gradually bends to converge with the plane of the tympanic membrane at its proximal end, producing a large yet flat recessus meatus, previously noted in Nandinia (Wible and Spaulding, 2013). The medial osseous external acoustic meatus near the recessus is thickened and somehow fused with the neighboring bulla wall in all these feliforms. The fused bulla near the recessus meatus is externally marked by the aforementioned depression (2.2.6) along the anterolateral corner of the bulla medial to the meatus.
On the ventral part of the centrifugal surface of the medial osseous external acoustic meatus, a triangular bony plate is observed in bilateral bullae, which is named here as the medial parameatal crest (pcm in Figs. 6, 8, 9). The medial parameatal crest has a long free posterior edge, a short dorsal edge running on the centrifugal meatal surface perpendicular to the adjoining crista tympanica and the longest anterior edge running from the borderline of the centrifugal meatal and bullar walls in a ventromedial direction on the internal surface of the “steep wall”. The meatal edge is about 7 mm long and the free edge is 11 mm. Lateral to the described medial parameatal crest lies a similar crest, termed the lateral parameatal crest here (pcl in Fig. 6), the meatal edge of which is much shorter and the anterior bullar edge of which runs ventrolaterally by contrast. This ventrolaterally running lateral parameatal crest is only a faint wrinkle in Dinocrocuta, while in Adcrocuta and Pachycrocuta it is well developed. Comparatively, the ventromedially running medial parameatal crest is absent in the latter two genera. No parameatal crest is discerned in extant hyaenids. In Barbourofelis loveorum, a ventromedially running medial parameatal crest appears to be present (Hunt, 1987:fig. 9).
Fig. 9
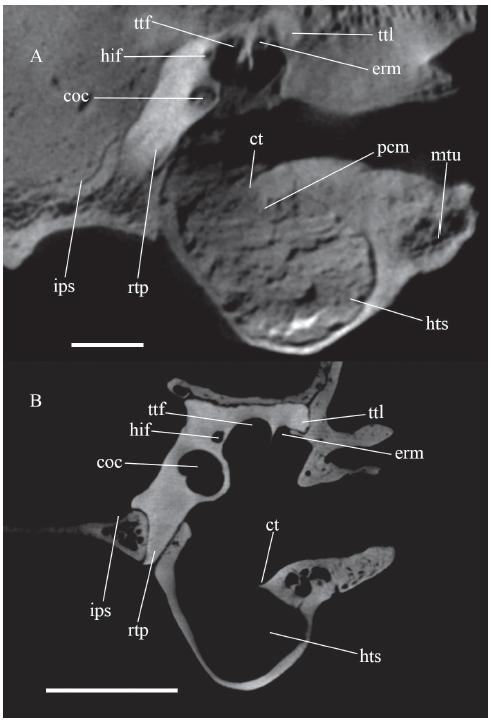 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 9Coronal section of left auditory region across the external acoustic meatus at the anterior part of the epitympanic recess
A. Dinocrocuta gigantea IVPP V 15649;B. Proteles cristatus IVPP OV 197
Abbreviations: coc. cochlea; erm. medial depression in the epitympanic recess roofed by the tegmen tympani; hif. hiatus Fallopii at its proximal end from cavum supracochleare; hts. hypotympanic sinus in the anterior chamber; ips. inferior petrosal sinus sulcus; mtu. meatal tubercle; rtp. rostral tympanic process of petrosal;ttl. lateral end of tegmen tympani pressing into the squamosal. Other abbreviations see Figs. 6, 8
Scale bars = 1 cm
2.3.2 Internal structures on the roof of anterior chamber
Glaserian fissure and the absence of the mallear hook A small recess is located anterodorsal to the supposed tympanic membrane (pars tensa) between the medial osseous external acoustic meatus and the roof of the anterior chamber, which could be regarded as a lateral diverticulum of the anteromedial recess beyond the “flat” (adr in Fig. 8). Further anterodorsal to this recess, the front wall of the bulla, presumably the expanded anterior crus of the ectotympanic, inflects upwards and backwards, forming a thick wall that lies against the epitympanic wings of the squamosal and alisphenoid with an angle of 70° to the basicranial plane. The fissure between the front wall and the appressed epitympanic wings is the Glaserian fissure. Owing to the anterior encroachment of the bulla, the area of the epitympanic wings of the squamosal and alisphenoid is considerably large in all examined feliforms except in the juvenile Paguma.
According to Wible and Spaulding (2012), a portion of the rostral process of malleus is anchored in the Glaserian fissure in juvenile carnivorans and fused to the dorsal surface of the posterodorsally wrapped anterior crus of the ectotympanic as a part of the bulla, namely the tympanic plate of the malleus. In the fissure, the tympanic plate of the malleus is bounded medially by the rostral tympanic artery and laterally by the chorda tympani (Davis and Story, 1943; Wible and Spaulding, 2013). The canal for the former is visible in all feliform specimens under CT investigation, while the canal of the latter, also known as the iter chordae anterius or canal of Hugier, is much smaller in diameter, and only observed in the three smaller specimens scanned with a higher resolution (around 40 μm). Anteriorly, these two canals end at a notch, and the tympanic plate of the malleus usually ends in a spine, both of which are already described.
There are two visible canals in the Glaserian fissure of Dinocrocuta. The lateral one (Fig. 8: canal in cyan color) directs to the notch for the rostral tympanic artery and the chorda tympani nerve and is identified as the course of the artery. The medial one (Fig. 8: canal in purple color) points to the medial side of the enteglenoid protuberance at a short distance. Considering its position, it may be the hiatus for the lesser petrosal nerve, which is poorly documented in carnivorans.
Wible and Spaulding (2012) also noted that a mallear hook is developed posterior to the tympanic plate from the medial side of the rostral process of the malleus in many carnivorans. After the fusion of the tympanic plate with ectotympanic, the mallear hook would remain backwardly protruding on the dorsal margin of the anterior crus of the ectotympanic, anterolateral to the tensor tympani fossa, and anteromedial to the epitympanic recess. In addition to the records in Nandinia, Panthera, Felis, and Genetta mentioned by Wible and Spaulding, a prominent mallear hook is also testified in Paguma and Ichneumia in current CT observation. However, in extant Hyaena and Proteles, only a small posterior protrusion shorter than 1 mm is found along the dorsal margin of the anterior crus of the ectotympanic near the tiny canal for the rostral tympanic artery, which could be a vestigial hook. In Crocuta and Dinocrocuta, no such protrusion is found. It is likely that the mallear hook of hyaenids, if ever developed, is either too anteriorly located, covered by and fused with the ectotympanic, or too posteriorly located, lost together with the proximal malleus.
In the roof posterior to the dorsal edge of the front wall, the bone tissue becomes loosely organized, obscuring sutural information, so that the presence of a parietal exposure on the bulla roof, which is mentioned by Wible and Spaulding (2013) in Nandinia, could not be investigated in Dinocrocuta.
Petrosal and its rostral tympanic process In the CT observation of all feliform specimens, the petrosal distinguishes itself from neighboring structures by its much higher density with the exception of its mastoid portion (the paroccipital process of the petrosal), which is often porous and merges with the caudal entotympanic and squamosal after puberty. This density difference to a great extent contributes to the following investigation on the petrosal. Anterior to the level of the posterior rim of the external acoustic meatus, the promontorium of the petrosal roofs the anterior chamber. At the medial edge of the promontorium projects ventrally the rostral tympanic process of the petrosal, which was elaborated by Hunt (1989) as the ventral promontorial process, and already mentioned in the description of the round pit in front of the auriform process of the basioccipital. The projected process forces the surface of the promontorium to incline laterally, making the anterior chamber medially shallow and laterally deep. The process, having a ventral exposure in most feliforms, is still around 7 mm away from the basicranial plane in Dinocrocuta (rtp in Fig. 9). The aforementioned round pit, reaching near its peak, could be compared to this ventral exposure. A presumed rostral entotympanic lies between the front slope of the rostral tympanic process of the petrosal and the epitympanic wing of the basisphenoid. The rostral entotympanic is represented by a mass of loose bone surrounding a triangular hollow space (ret in Fig. 10), which is consistent with its approximate range in other examined feliforms. Its ventral border with other bullar elements is not visible, and the hollow space within it doesn’t extend more ventrally to the bone of the “perpendicular wall”.
Fig. 10
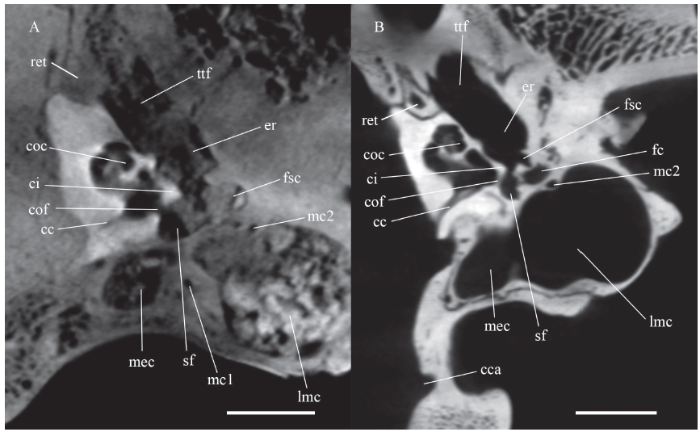 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 10Axial section of left auditory region slightly dorsal to the external acoustic meatus
A. Dinocrocuta gigantea IVPP V 15649; B. Crocuta crocuta IVPP OV 232, anterior to top, lateral to right
Abbreviations: cc. cochlear canaliculus; cca. condyloid canal at its posteroventral end; ci. crista interfenestralis; cof. cochlear fossula; fc. the third part of the facial canal near the foramen stylomastoideum primitivum;mc1. mastoid canaliculus at its segment dorsal to the middle constriced part of the posterior chamber;mc2. mastoid canaliculus at its distal end before joining the facial canal;ret. rostral entotympanic near its dorsal apex. Other abbreviations see Figs. 8, 9. Scale bars = 1 cm
The internal carotid artery enters the middle ear at the rear slope of the rostral tympanic process of the petrosal. It leaves no groove on the promontorium, and its entrance into the cranial cavity, the carotid foramen (middle lacerate foramen), is not found after careful search along the anteromedial corner of the bulla and the musculotubal canal. The complete obliteration of the carotid foramen has never been recorded in other feliforms (Pocock, 1916a; Davis and Story, 1943; Hunt, 1974, and personal observation).
Tensor tympani fossa Anterolateral to the promontorium lies the tensor tympani fossa (ttf in Figs. 8, 9), which constitutes part of the tegmen tympani, the bony laminar outgrowth of the petrosal. The fossa is almost in the shape of a standard hemisphere, facing ventrolaterally, the apex of which is the dorsalmost point of the entire middle ear lying 28 mm above the basicranial plane at the same height with the optic canal. Evolving a concave tensor tympani fossa is a trend in carnivoran evolution (Wesley-Hunt and Flynn, 2005). The depth of the fossa in Dinocrocuta is larger than those in Nandinia, felids, and viverrids (Jayne, 1898; Wible and Spaulding, 2012, and personal observation), almost the same as in Crocuta, slightly smaller than the ellipsoid fossae in Hyaena, Proteles (personal observation) and possibly Protictitherium (Fraile, 2015:fig. 7.1.8), and much smaller than the capsulate ones in herpestids (Hunt, 1991, and personal observation).
Epitympanic recess Dorsal to the crista tympanica and lateral and slightly posterior to the tensor tympani fossa lies the epitympanic recess (er in Figs. 8, 10), which is as long as the former but narrower. The roof of the epitympanic recess includes a medial petrosal portion, which is also part of the tegmen tympani, and a lateral squamosal portion. In the coronal section of the CT imaging (Fig. 9), the relationship between these two portions is clarified. The lateral end of the petrosal portion is rounded and swollen, pressing into the squamosal (ttl in Fig. 9). A ridge of the squamosal rises medially, wrapping the lower surface of the petrosal portion, and roofing the recess laterally. Posteriorly, the epitympanic recess is continuous with a shallow fossa incudis, which is excavated in the squamosal. As a result, the petrosal roof of the recess is deeper, forming a small medial depression (erm in Fig. 9).
The bipartite roof of the epitympanic recess is present in all feliforms examin-ed. However, the medial petrosal depre-ssion is observed in only extant hyaenids and the herpestid Ichneumia albicauda. In the juvenile Paguma and the tiger, such a depression is not apparent. Schmidt-Kittler (1981) noted a septum in the epitympanic recess dividing two depressions in some advanced mustelids, which seems to have evolved in parallel with the hyaenids and herpestids. These structures may be related to the dorsal morphology and superior suspensory ligaments of the malleus and incus.
Facial canal Conventionally, the facial canal is divided into three parts (van Kampen, 1905; van der Klaauw, 1931). The first part is from the internal acoustic meatus through the petrosal to its entrance into the middle ear cavity. The entrance is medial to the epitympanic recess and posterolateral to the tensor tympani fossa. In the middle of this part lies the cavum supracochleare, where the geniculate ganglion of facial nerve is located and the greater petrosal nerve branches off anteriorly through the hiatus Fallopii in the medial wall of the tensor tympani fossa (hif in Fig. 9). Owing to the dense petrosal enclosing this part, such configuration is clear in every observed feliform.
The second part is often referred to as the facial sulcus, where the facial nerve runs posterolaterally. In Dinocrocuta, the sulcus becomes indistinct posteriorly. Medial to the sulcus lie the fenestra vestibuli and the aperture of the cochlear fossula. The lateral side of the sulcus is the crista parotica. Ostensibly tiny and forgettable, it is a constant structure in the otic development of vertebrates (Moore, 1981) and is related to the formation of the tegmen tympani (Macphee, 1981). Moreover, the proximal end of the Reichert’s cartilage would be inserted on the lateral side of the crista parotica, part of which will later develop into the tympanohyal.
At the third part of the facial canal in extant hyaenids, the facial nerve bends at the foramen stylomastoideum primitivum, which is in front of the stapedius fossa and posterodorsal to the proximal end of the canal for the tympanohyal, runs ventrolaterally in an enclosed canal (fc in Fig. 10) parallel to the canal for the tympanohyal, and ultimately reaches the foramen stylomastoideum definitivum. This portion of the facial canal and the canal for the tympanohyal can’t be authentically reconstructed in Dinocrocuta. However, several stripes in the CT images that draw near the stapedius fossa ventrolaterally in the corresponding region suggest their presence and similarity with extant hyaenids.
Fossa for Spence’s cartilage Lateral to crista parotica occurs a rounded fossa (fsc in Figs. 8, 10) in Dinocrocuta. The fossa is located posteroventral and slightly medial to the fossa incudis, posterodorsal to the meatus, and anterior to the supposed region for the tympanohyal (inferred by the striped region mentioned above). It faces medially against and slightly anterior to the crista interfenestralis (ci in Fig. 10) with the facial sulcus located between them. A similar fossa is found in all feliforms observed, just anterior to the tympanohyal insertion on crista parotica. Wible and Spaulding (2013:44) described it as “the narrow, quadrangular posteroventral concavity of uncertain function” in Nandinia. The main difference of the fossa between Dinocrocuta and other feliforms is size. In Dinocrocuta, it is 3 mm anteroposteriorly long and 4 mm mediolaterally deep, while in Crocuta it is around 1 mm in diameter. In juvenile Paguma it is 1 mm long and quite shallow, similar to the one figured by Wible and Spaulding in Nandinia.
In soft tissue anatomy, Spence’s cartilage probably occupies this fossa. Sometimes referred as the caudal Chordafortsatz (in part) or the conical cartilage, it is recorded in felids, hyaenids, viverrids and herpestids (Spence, 1890; Bondy, 1907; Kamali et al., 2015). The Spence’s cartilage is rooted posteroventral to the fossa incudis, posterodorsal to the tympanic membrane, and guides the chorda tympani nerve to enter into the middle ear cavity. The chorda tympani nerve branches off the facial nerve from the third part of the facial canal and crosses the tympanohyal just through a canal, sometimes referred to as the canaliculus chordae tympani or iter chordae posterius, to enter the middle ear cavity (Wible and Spaulding, 2013), so the cartilage leading the nerve should be close to the tympanohyal, which is consistent with the embryological observation that it’s close to the Reichert’s cartilage (van der Klaauw, 1923). Therefore, the location of the Spence’s cartilage is identical with that of the observed fossa.
It’s noteworthy that in cats the cartilage reaches the promontorium at the crista interfenestralis (Khalil and Spector, 1985), which may relate it to another bony protrusion hanging from the crista parotica at the proximal end of the tympanohyal medially towards the crista interfenestralis, known as the mastoid tubercle in many carnivorans (Wang and Tedford, 1994; Westley-Hunt and Flynn, 2005). A distinct mastoid tubercle is not found in extant hyaenids or Dinocrocuta. In juvenile Paguma, the mastoid tubercle, contributed by both the petrosal and squamosal, is located slightly posterior to the supposed fossa for Spence’s cartilage, and medial to the ossified tympanohyal.
2.3.3 The Septum
The septum of the bulla roofs the posterior half of the anterior chamber and floors the posterior chamber. Laterally, it partially covers the stapedius fossa where the two chambers are connected. Its anterior margin abuts the promontorium (sep in Fig. 6) from the posterior limit of the aperture of the cochlear fossula anteromedially along the bulge on the promontorium that represents the first coil of the cochlea. Posteriorly, the septum slants downwards forming a 20° angle with the basicranial plane. The posterior margin of the septum where it joins the posterior wall of the bulla is located 12 mm dorsal to the basicranial plane, and 28 mm dorsal to the lowest point on the bulla floor, which means that the posterior face of the bulla is composed largely of the posterior wall of the anterior chamber (Fig. 7). When the exoccipital is in place, it conceals most of the area of the posterior bulla wall including all of that contributed by the posterior chamber. In many other hyaenids, the borderline of the two chambers on the bullar wall is located at or slightly anterior to the posteroventral edge of the bulla, ventral to the basicranial plane (De Beaumont and Mein, 1972; Hunt, 1974; Solounias, 1981; Qiu, 1987; Hunt and Tedford, 1993; Fraile, 2015), so that as a rule the posterior chamber wall would occupy most, if not all, of the area on the posterior face of the bulla in these hyaenids.
Posteromedially, the septum stretches into the boot-like process of the bulla dividing its internal space into a ventral invasion from the anterior chamber and a smaller dorsal invasion from the posterior chamber (bpa and bpp in Fig. 8). As is depicted by Solounias (1981), the anterior chamber doesn’t expand to the posteromedial corner of the bulla in many fossil hyaenids. According to the current CT observation, the boot-like process of the bulla in Crocuta is merely occupied by the posterior chamber as well. However, in Hyaena, Adcrocuta, and Pachycrocuta, the anterior chamber has evolved an incipient posteromedial invasion (different from the Hyaena bulla depicted by Solounias in his fig. 21E), somehow similar to the case in Dinocrocuta.
2.3.4 The posterior chamber
The posterior chamber lies dorsal to the septum. It is almost completely enclosed by part of the osseous complex of the bulla, presumably the caudal entotympanic, with the exception of a small promontorial exposure in front. Anteriorly the posterior chamber doesn’t exceed the contacting line of the septum and the promontorium. Transversely it can be divided into three parts, a medial compartment lying between the petrosal and the exoccipital (mec in Figs. 8, 10), a lateral mastoid cavity that inflates at the root of the mastoid process (lmc in Figs. 8, 10), and a middle constricted region (mic in Fig. 8).
Near the mastoid process, the septum curves ventrally reaching its lowest point about 5 mm ventral to the basicranial plane so as to floor the enlarged lateral cavity of the posterior chamber. This cavity is identical to the mastoid invasion or pocketing of the caudal entotympanic chamber in Herpestidae and Hyaenidae termed by Hunt (1987). In extant hyaenids and Ichneumia, the mastoid region is completely hollowed by this pocketing where the squamosal, petrosal and caudal entotympanic merge together. In Dinocrocuta, this invasion, termed the lateral mastoid cavity here, is medially more independent from the rest of the posterior chamber, and laterally limited to the proximal mastoid process. The bone tissue lining the chamber is condensed and compacted; thus, it prevents a connection from the cavity to the pores within the mastoid process. The lateral mastoid cavity, expanding along the mastoid crest, is 25 mm deep dorsoventrally. A hollowed mastoid process is also recorded in Tungurictis (Hunt and Solounias, 1991). In Protictitherium, the mastoid invasion is present, but the dorsolateral part of the mastoid process is still unhollowed (Fraile, 2015:fig. 7.1.7.4), which could be a primitive state in the Hyaenidae. The solid mastoid process of Dinocrocuta could be either derived from this state or a later reacquisition through a completely hollowed one.
The medial part of posterior chamber is principally a cavity located dorsolateral to the jugular foramen. This medial cavity, with a vertical height of 11 mm, is much smaller than the lateral mastoid cavity. Compared with extant hyaenids, the medial part is larger in Crocuta and smaller in Hyaena, both of which are also depicted by Qiu (1987:fig. 13). Two small crests are found at the ventromedial corner of the medial cavity in Dinocrocuta, and the narrow, long invasion into the boot-like process is from its anteroventral edge. Dorsal to it, the petrosal developed a small flat process, identical with the posterior apron of the petrosal mentioned by Hunt (1998).
At the junction between the medial and the middle constricted parts, the posterior chamber interconnects anteriorly with the anterior chamber through the stapedius fossa (sf in Fig. 8). The middle part is pressed from above, and around 5 mm in height. The bone pressing it seems to be a thickened and cancellous portion of the exoccipital that projects anteriorly and adjoins the pars canalicularis of the petrosal in front.
Mastoid canaliculus The mastoid canaliculus is found and reconstructed (Fig. 8: canal in red color) curling around the posterior chamber. It starts at the jugular foramen, ascends laterally across the posterior face of the medial cavity and arrives at the roof of the posterior chamber. Enclosed dorsally by the exoccipital and ventrally by the roof of the posterior chamber, it runs anteriorly along the borderline between the medial cavity and middle constricted part of the latter. Halfway on this borderline, it bends laterally on the roof of the middle part and approaches the borderline between the middle part and the lateral mastoid cavity (mc1 in Fig. 10) where it bends anteriorly again. Finally, the mastoid canaliculus diminishes at the anteromedial edge of the lateral mastoid cavity (mc2 in Fig. 10), which is the supposed region for the third part of the facial canal and is likely to join the canal there.
In extant hyaenids and herpestid Ichneumia, a similar mastoid canaliculus is found. Their posterior chamber is composed of a dorsoventrally deep mastoid cavity and a shallower medial part. The middle constricted region is not developed, so the lateral and medial counterparts are not as separated as in Dinocrocuta. Their mastoid canaliculus runs from the jugular foramen dorsolaterally to meet a parasagittal line that demarcates the medial and the mastoid cavity on the chamber’s roof where it bends anteriorly to join the third part of the facial canal that proceeds ventrolaterally. In felids and Paguma, a lateral mastoid inflation of the posterior chamber is not developed, and the mastoid canaliculus is less distinctly grooved on the caudal entotympanic, exoccipital and petrosal. It runs in a straight anterolateral direction rather than the sigmoid course in extant hyaenids and herpestids. Comparatively, the mastoid canaliculus of Dinocrocuta is much more sinuous and complex. According to the past anatomical studies (Gray, 1913; Davis and Story, 1943), the mastoid canaliculus houses the auricular branch of vagus nerve that branches off from the main trunk of the vagus nerve at the jugular foramen, runs dorsal to the bulla elements and joins the facial nerve to exit the cranium, as well as an accompanying artery that connects the stylomastoid artery to a branch of the inferior tympanic artery.
3 Discussion and conclusion
The large anterior chamber and the subhorizontal septum of Dinocrocuta show a typical hyaenid pattern (Hunt and Tedford, 1993). The posterior inflation of the anterior chamber already occurs in primitive hyaenids such as Tungurictis (Hunt and Solounias, 1991), Protictitherium (Fraile, 2015), Plioviverrops (De Beaument and Mein, 1972; Solounias, 1981) and Tongxinictis (Qiu et al., 1988b). All later hyaenids conform to this hyaenid pattern with the exception of Proteles (Solounias, 1981; Werdelin and Solounias, 1991) and a similar pattern occurs elsewhere only in the aberrant herpestid Cynictis (Hunt, 1987). In Stenoplesictis and felids, which were linked with Dinocrocuta by Chen and Schmidt-Kittler (1983), the septum is more vertical and the anterior chamber is restricted to the anterior or anterolateral part of the bulla (Hunt, 1998).The lateral mastoid cavity formed by lateral expansion of the posterior chamber also unites the Dinocrocuta with hyaenids and herpestids, but is not seen in felids or other Oligocene primitive feliforms. The sinuous route of mastoid canaliculus in Dinocrocuta, extant hyaenids, and herpestids is apparently associated with the development of the lateral mastoid cavity.
Besides the configuration of chambers, an inferior petrosal sinus sulcus lying on the intracranial face of basioccipital isolated from petrosal, a rostral process of malleus without a conspicuous spine of the tympanic plate or a mallear hook is found exclusively in Dinocrocuta and extant hyaenids among feliform groups. The hemispherical tensor tympani fossa of Dinocrocuta is deeper than that in Nandinia, viverrids, and felids, shallower than that in herpestids, but comparable with that in some extant hyaenids. The medial depression on the petrosal half of the epitympanic recess is observed in Dinocrocuta, extant hyaenids, and Ichneumia, but is absent in Panthera and Paguma. The phylogenetic significance of these features may be further revealed after a careful investigation on a larger coverage of fossil and extant taxa.
Some Dinocrocuta features occur only in some hyaenids: the constant presence of the alisphenoid canal is also recorded in some primitive hyaenids; a cylindrical external acoustic meatus composed of a meatal plate, which forms the anterior and dorsal wall, and a meatal tubercle, which floors the meatus is present in Pachycrocuta, Hyaena, and Crocuta; the deep fissure at the anteroventral edge between the meatal plate and the meatal tubercle in Dinocrocuta is probably homologous with an anteroventral meatal notch in ictitheres, Adcrocuta, and other carnivorans where the cylindrical meatus is not developed; some parameatal crests is formed on the centrifugal surface of the medial osseous external acoustic meatus near crista tympanica in Dinocrocuta as well as Adcrocuta and Pachycrocuta, though the crests differ in size and location among these genera; a massive, protrusive mastoid process is present only in Dinocrocuta and Chasmaporthetes. The muscular tubercle, the auriform process of basioccipital, and the boot-like process of bulla are present in some other bone-cracking taxa, though they are much more developed or distinct in Dinocrocuta.
The anteromedial part of the bulla of Dinocrocuta shows a series of unique structures, including the “flat”, the “perpendicular wall”, and the “steep wall”. A robust entoglenoid protuberance at the alisphenoid-squamosal suture and a middle constriction of the posterior chamber are previously unknown in Carnivora as well.
A fossa, present in all examined feliforms lateral to the crista parotica and presumably inserted by Spence’s cartilage, is much larger in Dinocrocuta than in other taxa.
Few features of Dinocrocuta bears resemblance exclusively to non-hyaenid feliforms. The roughened surface on the lateral half of the bulla is recorded elsewhere only in Barbourofelis. The complete loss of the condyloid canal occurs only in Dinocrocuta and a few felids.
In conclusion, the basicranial morphology of Dinocrocuta supports a hyaenid affinity, though a number of unique features argue that it could be an early side-branch within this family, as is suggested by Thenius (1966) based on the permanent dentition and chronological evidence.
This would, in turn, throw doubt upon the validity of Percrocutidae which was erected by Werdelin and Solounias (1991) according to the feature of dp4 demonstrated by Chen and Schmidt-Kittler (1983). The dp4 of Percrocuta and Dinocrocuta shows a feloid pattern in common with Stenoplesictis and felids where the metaconid moves backward and connects with the talonid with some ridges, while in other hyaenids the dp4 shows a viverroid pattern where the trigonid is well separated from the talonid by a deep transverse notch.
However, as is noted in the earlier work of Schmidt-Kittler (1976), the feloid pattern of dp4, i.e. backwards migrating metaconid and its close association with talonid could result from a rapid reduction of molars. In his investigation, this pattern occurs in not only Stenoplesictis, felids, Percrocuta and Dinocrocuta but also the nimravid Nimravus and a primitive carnivoran Palaeogale. In Wintershof-West materials of Semigenetta elegans, he observed both feloid and viverroid patterns. Moreover, the feloid pattern is recorded in the myrmecophagous hyaenid Proteles by Baryshnikov and Averianov (1995) who group it with Percrocuta and Dinocrocuta in a separate Protelidae. The dp4 of the euplerid Cryptoprocta, figured by Carlsson (1911), is probably similar to that of “Proailurus sp.” described by Chen and Schmidt-Kittler as an ancestral type of the feloid pattern. These taxa are unlikely to form a monophyletic group altogether, and the feloid pattern of dp4 must have evolved many times in different lineages of Carnivora, which is also the original conclusion drawn by Schmidt-Kitter (1976:99). Therefore, the shape of dp4 is not convincing enough as the only evidence of founding a separate family for Percrocuta, Dinocrocuta or Proteles. Given the basicranial morphology, Percrocutidae is probably invalid and could be relegated to Percrocutinae before the relationship between Percrocuta, Dinocrocuta and other hyaenids is better clarified.
Other than dental features, the only common feature of Percrocuta and Dinocrocuta is a short blunt angular process whose upper border is hardly curved. It is observed in the five Dinocrocuta gigantea mandibles available to me, including one associated with the cranium described here and studied by Tseng and Binder (2010), as well as Percrocuta miocenica (Chen and Schmidt-Kittler, 1983) and Dinocrocuta algeriensis (Arambourg, 1959). In the more primitive Percrocuta tobieni, the angular process seems more projected and slender (Crusafont-Pairó and Aguirre, 1971), which may mark the branching point of these two genera from other hyaenids with an ordinary angular process.
Acknowledgements
I thank Prof. Qiu Zhan-Xiang for offering constructive suggestions and comments, Mr. He Wen and Mr. Chen Shan-Qin for granting access to collections in Hezheng Paleozoological Museum, Mr. Hou Ye-Mao and Ms. Yin Peng-Fei for their help in Micro-CT scanning and reconstruction, thank the two reviewers, Dr. Wang Xiao-Ming and Dr. Ni Xi-Jun, for their comments and valuable suggestions. This work was supported by the National Natural Science Foundation of China (41430102) and the Chinese Academy of Sciences (XDB26000000, XDPB05, QYZDY-SSW-DQC022).参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 5]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 5]
[本文引用: 1]
[本文引用: 2]
[本文引用: 5]
DOIURL
PMID [本文引用: 1]

This study analyzed 76 species of Carnivora using a concatenated sequence of 6243 bp from six genes (nuclear TR-i-I, TBG, and IRBP; mitochondrial ND2, CYTB, and 12S rRNA), representing the most comprehensive sampling yet undertaken for reconstructing the phylogeny of this clade. Maximum parsimony and Bayesian methods were remarkably congruent in topologies observed and in nodal support measures. We recovered all of the higher level carnivoran clades that had been robustly supported in previous analyses (by analyses of morphological and molecular data), including the monophyly of Caniformia, Feliformia, Arctoidea, Pinnipedia, Musteloidea, Procyonidae + Mustelidae sensu stricto, and a clade of (Hyaenidae + (Herpestidae + Malagasy carnivorans)). All of the traditional "families," with the exception of Viverridae and Mustelidae, were robustly supported as monophyletic groups. We further have determined the relative positions of the major lineages within the Caniformia, which previous studies could not resolve, including the first robust support for the phylogenetic position of marine carnivorans (Pinnipedia) within the Arctoidea (as the sister-group to musteloids [sensu lato], with ursids as their sister group). Within the pinnipeds, Odobenidae (walrus) was more closely allied with otariids (sea lions/fur seals) than with phocids ("true" seals). In addition, we recovered a monophyletic clade of skunks and stink badgers (Mephitidae) and resolved the topology of musteloid interrelationships as: Ailurus (Mephitidae (Procyonidae, Mustelidae [sensu stricto])). This pattern of interrelationships of living caniforms suggests a novel inference that large body size may have been the primitive condition for Arctoidea, with secondary size reduction evolving later in some musteloids. Within Mustelidae, Bayesian analyses are unambiguous in supporting otter monophyly (Lutrinae), and in both MP and Bayesian analyses Martes is paraphyletic with respect to Gulo and Eira, as has been observed in some previous molecular studies. Within Feliformia, we have confirmed that Nandinia is the outgroup to all other extant feliforms, and that the Malagasy Carnivora are a monophyletic clade closely allied with the mongooses (Herpestidae [sensu stricto]). Although the monophyly of each of the three major feliform clades (Viverridae sensu stricto, Felidae, and the clade of Hyaenidae + (Herpestidae + Malagasy carnivorans)) is robust in all of our analyses, the relative phylogenetic positions of these three lineages is not resolvable at present. Our analyses document the monophyly of the "social mongooses," strengthening evidence for a single origin of eusociality within the Herpestidae. For a single caniform node, the position of pinnipeds relative to Ursidae and Musteloidea, parsimony analyses of data for the entire Carnivora did not replicate the robust support observed for both parsimony and Bayesian analyses of the caniform ingroup alone. More detailed analyses and these results demonstrate that outgroup choice can have a considerable effect on the strength of support for a particular topology. Therefore, the use of exemplar taxa as proxies for entire clades with diverse evolutionary histories should be approached with caution. The Bayesian analysis likelihood functions generally were better able to reconstruct phylogenetic relationships (increased resolution and more robust support for various nodes) than parsimony analyses when incompletely sampled taxa were included. Bayesian analyses were not immune, however, to the effects of missing data; lower resolution and support in those analyses likely arise from non-overlap of gene sequence data among less well-sampled taxa. These issues are a concern for similar studies, in which different gene sequences are concatenated in an effort to increase resolving power.
[本文引用: 5]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 4]
[本文引用: 10]
[本文引用: 6]
[本文引用: 4]
[本文引用: 3]
DOIURL [本文引用: 1]
[本文引用: 3]
[本文引用: 3]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

The small Indian mongoose (Herpestes javanicus) is native to the Middle East, Iran and much of southern Asia. For this study the middle ears of a total of 6 adult small Indian mongooses, both fresh and museum samples were explored by using of dissection and plain radiography. On the one hand, at least in some species of the mongoose vocalisations and hearings play a critical role in coordinating behaviours. On the other hand, the ear region has provided useful character relevant for mammalian phylogeny. So, the aim of the present study is a brief discussion of the various anatomic particularities of the middle ear based on a combination of existing data and the results of the authors' study in the small Indian mongoose.
PMID [本文引用: 1]

The object of this study was to investigate the anatomy, histology, and possible function of a conical structure found in the middle ear of the cat. This conical structure lies across the dorso-caudal compartment of the middle ear. It is directly related to the course of the chorda tympani nerve in the middle ear. Its base is attached by fibrous tissue to the dorso-caudal segment of the tympanic bone adjacent to the tympanic membrane. Its apex rests on the promontory just rostral to the round window niche. Histologically, it is cartilaginous tissue enveloped by a mucous membrane with no trace of bone. Portions of some conical cartilage specimens display extensive calcification. A previous hypothesis suggests that this structure conducts high frequency sounds directly from the tympanic membrane to the round window membrane. This seems unlikely because its length is shorter than the distance between the tympanic membrane and round window membrane. The conical cartilage may be a vestigial remnant of the second arch bar (Reichert's cartilage).
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 3]
[本文引用: 2]
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 4]
DOIURL [本文引用: 1]
PMID [本文引用: 1]

The musculature of the neck and the forelimb of Hyaena hyaena is described and the biomechanical implications of some morphological aspects of muscles and skeleton are discussed. The extensors of the head and neck, the protractors and retractors of the forelimb and the extensors of the shoulder, elbow and carpal joints are relatively stronger developed than those in Canidae and Felidae. Moreover these muscles have larger moments about the joints. This is considered as an adaptation to lifting and carrying large and heavy prey or carrion.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 5]
DOIURL [本文引用: 2]
[本文引用: 4]
DOIURL [本文引用: 10]
[本文引用: 3]
