 ,1,2,*
,1,2,*A preliminary study of serial stable isotope analysis tracks foraging ecology of fossil Asian elephants in South China
MA Jiao1,2, WANG Yuan1,3, JIN Chang-Zhu1, ZHANG Han-Wen4,5, HU Yao-Wu ,1,2,*
,1,2,*通讯作者: *ywhu@ucas.ac.cn
收稿日期:2019-01-4网络出版日期:2019-07-20
| 基金资助: |
Corresponding authors: *ywhu@ucas.ac.cn
Received:2019-01-4Online:2019-07-20

摘要
为了进一步探索亚洲象的摄食行为,运用稳定同位素的序列取样(serial/sequential sampling)新方法,首次对晚更新世笆仙洞遗址的三个亚洲象臼齿牙釉质(1个DP4, 2个M1)进行研究。结果表明,3个亚洲象个体的δ13C和δ18O内部差异均很小,未见季节性变化,虽然可能存在断奶及迁徙导致的数据波动,但总体来看依然表现出在牙釉质形成的长期过程中较为稳定的摄食行为。之前笆仙洞亚洲象动物群的整体取样(bulk sampling)同位素研究结果中,亚洲象的数据分布较为分散。而本次研究中较小的个体内部差异,则反向证实了宽泛分布的数据确实代表了灵活的摄食行为,并非取样位置的不同所致。这也进一步证明在气候温暖的东南亚地区,长鼻类动物的牙釉质整体取样工作可以提供可靠的古摄食行为及古生态信息。
关键词:
Abstract
Until now, feeding ecology has been found to play a significant role in the evolution of Asian elephant Elephas maximus. As the most widely-applied method in this field, bulk stable isotope analysis on tooth enamel had revealed important evidence on their paleodiet and paleoecology. However, it might be not skilled at reflecting the overview of the paleoecology of elephants, considering their huge tooth mophology and long dental ontogeny process. A newly-developing serial sampling strategy on tooth enamel sections could provide an effective approach to reconstruct the long-term individual life history of mammals covering the whole tooth formation time with higher precision. In this study, serial sampling isotope analysis was firstly undertaken on tooth enamel of Asian elephants from Baxian Cave, South China during the Late Pleistocene. The within-tooth isotopic variations of three teeth (one DP4 and two M1s) are all surprisingly subtle (standard deviations of δ 13C and δ 18O values are all less than 0.6‰), though some obvious variations might be caused by weaning and/or possible migration. No seasonal variation was observed, possibly indicating that these elephants had a stable foraging ecology. Back to our previous bulk tooth enamel isotope analysis on this same site, we could confirm that the varied bulk isotope results of Asian elephants factually represent their flexible foraging ecology. We may thereby conclude that the increasing bulk isotopic analysis in this region can provide a reliable paleoecological proxy for Pleistocene proboscidea in the warm regions of South and Southeast Asia.
Keywords:
PDF (2409KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
马姣, 王元, 金昌柱, 张瀚文, 胡耀武. 序列取样的稳定同位素研究示踪中国晚更新世亚洲象的摄食行为. 古脊椎动物学报[J], 2019, 57(3): 225-240 DOI:10.19615/j.cnki.1000-3118.190327
MA Jiao, WANG Yuan, JIN Chang-Zhu, ZHANG Han-Wen, HU Yao-Wu.
1 Introduction
The Asian elephant is one of the largest terrestrial animals in the world today, and is now an endangered species sporadically distributed in South and Southeast Asia (Maglio, 1973; Shoshani and Eisenberg, 1982; Shoshani and Tassy, 1996). They possess highly-evolved hypsodont molars with strong chewing ability, which are suggestive of adapting to a diet dominated by abrasive vegetation (Maglio, 1973; Sukumar, 2006; Sanders, 2018). It is generally believed that modern Elephas maximus is broadly speaking, a mixed browser and grazer, with a large dietary breadth depending on seasonality and geography (Sukumar and Ramesh, 1995; Cerling et al., 1999; Pradhan et al., 2008; Ahrestani et al., 2016; Baskaran et al., 2018a). However, based on the previous stable isotopic data, their diet had varied greatly from C4 to a C3-dominated vegetation during their evolutionary history (Cerling et al., 1999; Patnaik et al., 2014; Ma et al., 2017; Patnaik, 2017).Even though the bulk tooth enamel isotopic analysis has been effectively applied on fossil mammals for several decades, it still has some intrinsic limitations on megaherbivores. Of particular note here is the fact that elephantid molars are morphologically huge and formed during a long and continuous process of dental ontogeny (Laws, 1966; Roth and Shoshani, 1988; Hillson, 2005; Sanders, 2018). Some researchers suggested to obtain bulk samples covering the whole range of tooth height to average the different results (Pederzani and Britton, 2019), but it is not practical for elephants considering the damage to the specimen and the contaminations on the whole surface. Therefore, it is possible that the stable isotopic ratios obtained from a small portion of bulk tooth enamel could be influenced by the different sampling loci on a tooth, which may cause misleading dietary interpretations, also as mentioned in some case studies (Feranec and MacFadden, 2000; Hoppe and Koch, 2006; Pederzani and Britton, 2019).
By contrast, serial sampling of tooth enamel provides an effective approach to reconstruct the long-term dietary and ecological use of mammals, during the time of tooth growth with higher precision (Fox and Fisher, 2004; Zazzo et al., 2006; Fox et al., 2007). This method is suitable for the huge molars of proboscidea and has been successfully applied on extinct mammoths and mastodons to track individual life history for signatures related to climate change, seasonal dietary shifts, and tooth enamel growth rate among other scientific questions (Koch et al., 1998; Feranec and MacFadden, 2000; Hoppe and Koch, 2006; Metcalfe and Longstaffe, 2012, 2014).
During the Late Pleistocene, E. maximus was widespread across South and Southeast Asia (Shoshani and Eisenberg, 1982; Shoshani, 1998), and also was the characteristic mammal in South China with increasing materials in recent years (Wang et al., 2017a, b; Tong et al., 2018). In our previous study (Ma et al., 2017), bulk stable isotope (C, O) analysis on Asian elephants fauna during that time from the Baxian Cave, Guangxi, China, supplied the missing link of Asian elephants foraging ecology during the Late Pleistocene. The isotope results of Asian elephants were widely distributed among the whole fauna in pure C3 environment, indicating a varied diet. However, based on the consideration aforementioned, these variations could also be influenced by weaning, dietary seasonality, or intraspecific difference due to the bulk sampling strategy (Ma et al., 2017).
Herein, we present a first case study of serial sampling on the tooth enamel of fossil Asian elephants from Baxian Cave. Serial stable isotope (C, O) analysis was preliminarily undertaken with the aim to further understand the isotopic variability during the tooth growth period covering the premolar and molars. Some important information on nursing, dietary seasonality, and intraspecific difference of elephants will be discussed, alongside other potential factors which may explain better about their long-term foraging ecology.
2 Methodology
Species of the Elephantidae are different to all other animals in having high and thick molar crown, composed of numerous tooth plates (Maglio, 1973; Sanders, 2018). Like other derived elephantids, Elephas maximus has six teeth in each quadrant and 24 teeth in total (Roth and Shoshani, 1988; Sanders, 2018). The molars are horizontally developed from the back of the jaw and successively replaced one by one, two of which at most could be in use synchronously in each quadrant (Shoshani and Eisenberg, 1982; Roth and Shoshani, 1988; Sanders, 2018). In general, the six teeth in one quadrant of whole tooth row include three deciduous premolars (DP2, DP3, and DP4) and three molars (M1, M2, and M3) (Laws, 1966; Shoshani and Tassy, 1996).Currently, there has been no study focusing on the determination of Asian elephant tooth formation age and enamel growth rate reported yet. However, given the substantial dental morphological similarity and close phylogenetic relatedness between E. maximus and Late Pleistocene mammoths (Mammuthus spp.) (Shoshani and Eisenberg, 1982; Shoshani and Tassy, 1996; Roca et al., 2015; Sanders, 2018), we may hereby reasonably assume that the data summarized form modern African elephants (Laws, 1966) provide suitable background references for previous study on mammoth (Metcalfe, 2011; Metcalfe and Longstaffe, 2012), and also E. maximus in this study. Here, we infer the DP4 and M1 as being roughly developed during the time of pre-birth to 3 years old and 3-15 years old respectively (Metcalfe et al., 2010; Metcalfe, 2011), and the enamel extension rate of teeth plates following the height direction is the 13-14 mm/yr (Metcalfe and Longstaffe, 2012). This fundamental basis is quite vital to estimate the approximate age of the animal during any apparent dietary or ecological change as recorded in the enamel serial sections.
The individual plates which make up an elephant tooth develop vertically to tooth occlusal plane, with the oldest dental tissue located towards the occlusal surface and the youngest towards the root. The stable isotope (C, O) ratios of serial sections sampled on the tooth enamel thus record the specific foraging behavior of the individual during the formation time of the different tooth sections. Therefore, the isotopic profile of the serial sections of tooth enamel may indicate dietary and habitat shifts of individual elephants during different ontogenetic phases of each tooth. In combination with the aforementioned growing parameters of tooth enamel, a lot of important information, such as those which may elucidate climatic change and seasonal dietary shifts, possibly be recorded through the isotopic signatures of proboscidean teeth (Koch et al., 1998; Feranec and MacFadden, 2000; Hoppe and Koch, 2006; Metcalfe and Longstaffe, 2012, 2014). Therefore, this first preliminary serial sampling isotope work on E. maximus dentition will have the potential to effectively elucidate shifts in their foraging ecology through life history, and also help recheck the results from different sampling strategies.
3 Materials and methods
3.1 Geological setting of fossils and sample selection
Baxian Cave (22°34′31.6″N, 107°21′0.2″E) was systematically excavated by the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences (IVPP). It is located at the town of Zuozhou, Chongzuo, Guangxi in South China. Baxian Cave and its adjacent areas are characterized by a bare karst landscape, with a northern tropical climate. The sediments from Baxian Cave are approximately 5 m thick, and can be divided into five layers from top to bottom. Three elephant fossils in this study and other associated mammals in previous study were all unearthed from the third layer, indicating they are contemporary (Ma et al., 2017).After systematic excavation, a large variety and number of vertebrate fossils belonging to at least 40 large-mammalian species were unearthed from the deposits in Baxian Cave, including extremely abundant fossil teeth of the Asian elephant and other associated mammals. Based on the similarity with those nearby faunal assemblages and deposits, which had already obtained the accurate dating age, such as Zhiren Cave, Chongzuo, dated to 100-113 ka (Jin et al., 2009; Liu et al., 2010) and Fuyan Cave, Daoxian, Hunan, dated to 80-120 ka (Liu et al., 2015), the geological age of Baxian Cave possibly also belong to the early Late Pleistocene. The dating of U-series and Electron Spin Resonance Dating (ESR) to determine the absolute date of fossil occupation will be published elsewhere with other taxonomy materials.
Three Elephas maximus teeth, including one upper right DP4 (IVPP V 22700.01) and two M1s (V 22700.02 with only the median portions preserved; and V 22700.03 with natural front end preserved as demarcated by the presence of the anterior talon), were selected here for serial sampling. These three teeth belong to different individuals. The tooth plate with the longest in length and well-preserved condition of each elephant tooth was chosen for sequential sampling. These are the 8th preserved lamella on V 22700.01 (labial side), the 3rd preserved lamella on V 22700.02 (labial side), and the natural 8th lamella on V 22700.03 (labial side). All three sampled teeth are well preserved based on the preservation assessment of teeth apatite by the analyses of X-ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) (Ma et al., 2017).
3.2 Sequential sampling of teeth and stable isotope measurements
The selected tooth plates and the sequential sampling positions are illustrated in Fig. 1. First, contaminants adhering to the surfaces of the chosen lamellae were carefully cleaned off with a diamond-tipped dental burr. Second, the drill lines were perpendicular to crown height, the intervals moved sequentially up the crown height at intervals of approximate 1-2 mm, measured by vernier calliper. The sampling strategy was designed to cover the entire crown height of each sampled lamella as much as possible. However, some upper and lower parts of the tooth plate were not sampled to avoid the possible contamination caused by the frequent contacts of the crown and cervix with the sediments (Fig. 1). Finally, 24, 46, and 54 serial samples from V 22700.01, 02, and 03, were obtained respectively, covering the lengths of 41.2, 92.8, and 72.1 mm respectively between the apex of the tooth crown and the cervix.Fig. 1
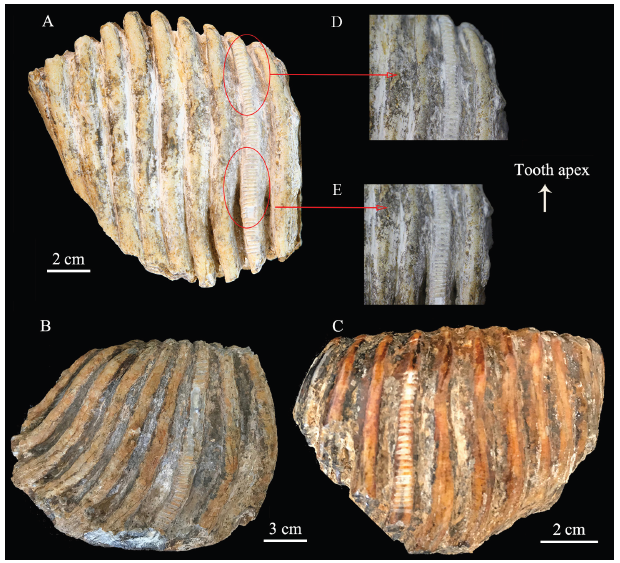 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 1Diagrams of serial sampling on tooth enamel of Elephas maximus
A. IVPP V 22700.03; B. V 22700.02; C. V 22700.01;D, E. the partially enlarged sampling details from V 22700.03
Protocols for the preparation of collected enamel powders followed Lee-Thorp et al. (1989) with some modifications, same to the protocol in Ma et al., 2017. Given the fact of weight losses to the enamel powders after the preparation procedures, two or three consecutive serial samples were combined into one in order to reach the requirement of sample weight for stable isotope measurements. After the reorganization, 13, 35, and 23 samples for V 22700.01, 02, and 03 were obtained.
Here, an Isoprime 100 Isotope Ratio Mass Spectrometer (IRMS), coupled with a multi-flow system, was used to measure carbon and oxygen isotopic ratios at the Archaeology Stable Isotope Laboratory in the Department of Archaeology and Anthropology, University of Chinese Academy of Sciences. The bioapatite powder of each sample with the weight of about 2 mg was packed into sealed glass tubes and flushed with high-purity helium. Then 0.6 ml of ultrapure phosphoric acid (H3PO4) at 70°C was injected into every tube using a disposable medical injector. After the one-hour reaction maintained at 80°C, the carbon dioxide released in the tube was eventually conveyed by helium as carrier gas to the IRMS. International standards, IAEA CO-8 (standard δ13C value: -5.8‰; standard δ18O value: -22.7‰) and IAEA-603 (standard δ13C value: 2.5‰; standard δ18O value: -2.4‰), were used for isotopic calibration and inserted after every ten samples. In addition, another international standard of NBS18 (standard δ13C value: -5‰; standard δ18O value: -23.2‰) was inserted as well randomly as reference for monitoring the measurement stability. The long-term measurement precisions were better than ± 0.2‰ for both δ13C and δ18O values. Their isotopic data were listed in Supplementary Tables 1-3.
4 Results
The isotopic profiles of the sampled three elephant teeth are showed in Fig. 2. In summary, the δ13C values of the whole isotopic data range from -18.3‰ to -16.3‰, while the δ18O values range from -7.9‰ to -4.2‰. According to the δ13C enrichment (14.1‰) from diets to bone or tooth apatite for large herbivores (Cerling and Harris, 1999), the δ13C values of their diets range from -32.4‰ to 30.4‰, indicating a consumption of entire C3 plants. The ?13C values for V 22700.01, 02, and 03, representing the isotopic difference between the maximum and minimum, are 1.3‰, 1.6‰, and 1.0‰ respectively; and the respective ?18O values are 1.7‰, 2.0‰, and 2.0‰. Therefore, the within-tooth isotopic variation in each tooth is small. The details on the isotopic profiles of each tooth are presented as follows.Fig. 2
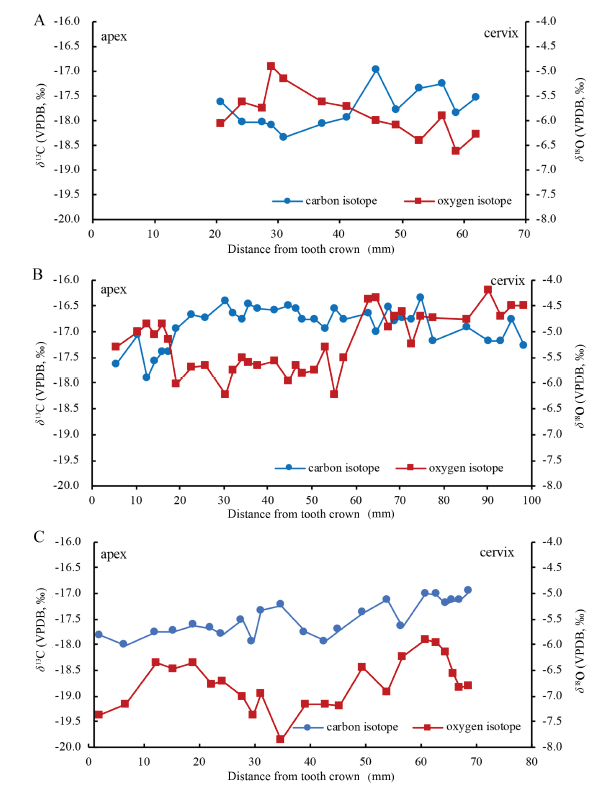 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 2Serial analysis results of δ13C and δ18O values of the sampled three elephant teeth of Elephas maximus
A. IVPP V 22700.01; B. V 22700.02; C. V 22700.03
The δ13C values of V 22700.01 (DP4) range from -18.3‰ to -17.0‰, yielding an average of (-17.8 ± 0.4)‰ (n=13), while the δ18O values range from -6.6‰ to -4.9‰ with the mean of (-5.9 ± 0.5)‰ (n=13). As illustrated in Fig. 2A, the δ13C values remain relatively stable, reach the highest at the eighth point and fluctuate to some extent in the profile of δ13C values. For the δ18O values, the highest peak is observed at the fourth point and then decrease gradually.
The δ13C and δ18O values of V 22700.02 (M1) range between -17.9‰ to -16.3‰ and -6.2‰ to -4.2‰, averaging at (-16.9 ± 0.4)‰ (n=35) and (-5.2 ± 0.6)‰ (n=35) respectively. The δ13C values reach the lowest at the third point, then gradually rise towards a higher level from the seventh point to the peak at the 29th point, and then decline slightly afterwards. The δ18O values exhibit a more complex pattern than δ13C values. Higher δ18O values are seen during the range of first six points, then drop sharply and keep relatively constant from the seventh to 22nd point. The rapid increase of δ18O values can be seen from the 21st to 23rd loci and maintain higher level afterwards until the peak at 32nd point.
For molar V 22700.03 (M1), the sampled lamella is measured between 2-68.7 mm from tooth crown, corresponding to more than half length of this tooth plate (~110 mm). The δ13C values range from -18.0‰ to -17.0‰, with the average of (-17.5 ± 0.3)‰ (n=23) while the δ18O values range from -7.9‰ to -5.9‰, on an average of (-6.8 ± 0.5)‰ (n=23). In brief, the δ13C values fluctuate to small scale and increase gradually, while the lowest δ18O values are observed in the middle point while the high values are around the two ends.
5 Discussions
The δ13C average of the three individual samples are quite similar as -17.8‰, -16.9‰, and -17.5‰, well corresponding to the previous bulk sampling isotope results (Ma et al., 2017). Nevertheless, we found there are indeed some notable isotope variations in the profiles of tooth sections for each sampled tooth (Fig. 2). Several possible factors could account for these within-tooth variations, such as weaning and seasonal effects.The oxygen isotopic values of infant mammalian tissue samples during the milk-feeding stage would be increased compared to their mothers, due to their consumption of milk, which contains higher δ18O values (Wright and Schwarcz, 1998, 1999; Renou et al., 2004; Britton et al., 2015; Tsutaya and Yoneda, 2015). Once weaning takes place and eventually milk consumption stops, the δ18O values of infants will drop gradually and reach to the same values as the mothers (Wright and Schwarcz, 1998; Britton et al., 2015). For modern Asian and African elephants, the nursing period may at least last two years to meet the nutritional demand of infant elephants, even last up to six to eight years long until the birth of a sibling (Lee and Moss, 1986; Lee, 1996; Sukumar, 2003; Wittemyer et al., 2007a). Based on the aforementioned tooth formation age (Metcalfe et al., 2010; Metcalfe, 2011), tooth enamel growth rate (Metcalfe and Longstaffe, 2012), and the tooth enamel wear on crown surface, the decline potential of δ18O values on the three samples (these three decline locus are respectively 29, 16.1, and 19 mm away from their tooth crown) seemingly all fall into the range of their weaning age, thus could be possibly influenced by weaning effect. Nevertheless, future research on the tusk samples of fossil elephantids would probably provide more accurate evidence on nursing and weaning than from check teeth samples, as indicated by prior works done on Late Pleistocene mammoths (Rountrey et al., 2007; Cherney, 2016).
Seasonal foraging preferences on different plants during wet and dry seasons have been widely found in the modern Asian elephant (Sukumar, 1989, 2003, 2006; Sukumar and Ramesh, 1995; Chen et al., 2006; Baskaran et al., 2010; Roy, 2010; Mumby et al., 2013) and African elephant (Barnes, 1982; Koch et al., 1995; Cerling et al., 2004, 2006, 2009; Codron, 2004; Wittemyer et al., 2009; Codron et al., 2012, 2013; Forrer, 2017) living in tropical regions. However, no regular significant fluctuations of the isotopic data are found in present study (Fig. 2). In the pure C3 environments, the slightly seasonal variation of the vegetation ingested by herbivores might be hard to detect by δ13C values. However, the δ18O values of the large mammals in this region could be potentially influenced by seasonality, where the monsoon effect cause big seasonal variations on δ18O values of local precipitation and then the drinking water of the large-sized obligate drinkers (Bryant and Froelich, 1995; Dutton et al., 2005; Biasatti et al., 2010). However, no seasonal variations were observed on δ18O values of all these three samples, it might because that these elephants drank from the standing water bodies, such as lakes, where the seasonal δ18O values are largely buffered due to long water residence times (Pederzani and Britton, 2019). Therefore, the other sharp increase or decrease of isotopic signatures (except for the aforementioned δ18O values declines possibly suggestive of weaning) (Fig. 2), is much likely caused by possible movements to new habitats, where elephants ingested plants or water of different stable isotopic properties.
All in all, the isotopic variations restricted from 1‰ to 2‰ among each elephant tooth sections found in this study could be explained, in theory, by the weaning and/or migrational effects. Nevertheless, these isotopic variations within each of the three sampled tooth enamel sections are still generally small. The standard deviations for both δ13C and δ18O sequential values of each tooth are overall less than 0.6‰. It may thus be concluded that, these Asian elephants all inhabited in a relatively dense and stable forested environment during the Late Pleistocene, without obvious age and seasonal difference.
Several case studies of stable isotope analysis on modern Asian elephants in India and Thailand displayed a wide range of δ13C values, representing both C3 and C4 food ingestion (Sukumar and Ramesh, 1992; Pushkina et al., 2010; Roy, 2010). .Whereas, plenty of isotope analysis had been applied on modern African elephants for the purpose of tracing ivory trade and conservation biology, which also displayed mixed C3 and C4 diet, and higher seasonal variabilities among their isotopic profiles of tusks and hair sections (van der Merwe et al., 1990; Vogel et al., 1990; Cerling et al., 2004, 2006, 2009; Wittemyer et al., 2009; Codron et al., 2013). This further highlights the feeding flexibility and opportunism of elephantids from an evolutionary perspective (Cerling et al., 1999; Sukumar, 2003; Ma et al., 2017; Wang et al., 2017a; b; Zhang et al., 2017; Wu et al., 2018), and may demand more comparable evidence from their modern and fossil counterparts, and also from more regions, to better understand the interaction between ecological evolution and extinction/survival.
Back to the aforementioned consideration about possible bias caused by different bulk sampling loci on elephant teeth, this study could now exclude the seasonal effects. Based on the limited data variations, it is also reasonable to look beyond within-tooth isotopic variations caused by weaning and possible migration. Considering the increasing number of stable isotopic studies on bulk enamel from mammalian remains in South and Southeast Asia (Qu et al., 2014; Ji et al., 2016; Bocherens et al., 2017; Li et al., 2017; Suraprasit et al., 2018; Bacon et al., 2018a, b), especially our continuing exploration on foraging ecology of other proboscideans in Quzai Cave, South China (Ma et al., in review), this serial isotopic work provides significant evidence that the widely applied procedure can provide reliable inference of foraging ecology from the duration of mammalian tooth growth in warm temperate regions, unlike the evidence from high-latitude regions (Feranec and MacFadden, 2000). More similar studies on E. maximus fossils from wider area will be useful to reveal the foraging complexity and flexibility of Elephas maximus in this region, and would also be a reference to conserve this highly endangered animal today.
Finally, as the typically gregarious mammal (Jayantha et al., 2009), modern Asian elephants in India was surprisingly found that, their social structure played a significant role in habitat selection except for extrinsic environmental factors (Baskaran et al., 2018b). This phenomenon was also found previously on African elephants (Wittemyer et al., 2007b), in combination with genetic study on mammoth (Pe?nerová et al., 2017), suggesting the significance of their social structure, also as warned by Fisher in the review article about the paleobiology of Pleistocene proboscideans in 2018. Our previous bulk isotope analysis of Asian elephants had disparity as two different groups. Even though weaning, seasonality, and other possible factors had been excluded by this study, it is still unconvincing to say this disparity was influenced by different ecological occupation of different elephant herds. However, at least it emphasizes and reminds us again to take social structure into consideration when reconstructing the paleoecology of fossil proboscideans. Furthermore, this provides a novel independent line of evidence which may potentially provide further corroboration on the complex metapopolational phylogeographic history of E. maximus, as highlighted by previous mitochondrial DNA studies (Fernando et al., 2000; Vidya et al., 2005, 2009; Yang and Zhang, 2012; Girdland-Flink et al., 2018; Kusza et al., 2018), and opens up a new window for reconstructing Late Pleistocene proboscidean paleobiology in South Asia.
6 Conclusions
In this study, serial sections of tooth enamel from three Asian elephant specimens from the Late Pleistocene deposits of Baxian Cave, Guangxi, China, were conducted for stable isotopic analysis (C, O). Their average δ13C values are (-17.8 ± 0.4)‰ (n=13), (-16.9 ± 0.4)‰ (n=35), and (-17.5 ± 0.3)‰ (n=23), respectively; whereas their respective mean of δ18O values are (-5.9 ± 0.5)‰ (n=13), (-5.2 ± 0.6)‰ (n=35), and (-6.8 ± 0.5)‰ (n=23). The isotopic profiles of three elephant teeth thus fluctuate to some degree, possibly caused by weaning and/or migration. The most important finding here is that the isotopic variability existing within the tooth profiles are highly constrained without notable signal from seasonal effect and other potential factors, suggesting a stable foraging ecology for the Asian elephants in the presently studied area during the Late Pleistocene. This isotopic pattern is different from other elephantids, such as the extinct mammoth Mammuthus spp., and the extant African elephant Loxodonta africana. Besides, this study here confirms that the widely-used bulk stable isotope analysis could supply reliable reference about their foraging ecology in this region without obvious interference from several possible factors. It also evidences the ecological flexibility of Asian elephants revealed by our previous bulk isotope analysis and emphasizes again the ecological significance of their social structure. More similar studies on both modern and fossil Asian elephants from wider area in South and Southeast Asia in the future will be useful to reveal if this apparent past and present pattern of foraging complexity and flexibility in Asian elephants continue to hold true.Acknowledgements
We are very grateful for the hard work of LIU Yi-Hong, ZHANG Ying-Qi, and ZHU Min from IVPP who attended the fieldwork at Baxian Cave. Additionally, we appreciate all the help from the bioarchaeology research group in the Department of Archaeology and Anthropology in University of Chinese Academy of Sciences. We are particularly grateful for the technical assistance on the Mass Spectrometer from Dr. WANG Ting-Ting in Sun Yat-sen University and the valuable suggestions and revisions from the reviewer Prof. DENG Tao and the editor Dr. ZHOU Shuang.Supplementary material can be found on the website of Vertebrata PalAsiatica (
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 2]

?The isotope enrichment ?* of C between tooth enamel of large ruminant mammals and their diet is 14.1?±?0.5‰. This value was obtained by analyzing both the dental enamel of a variety of wild and captive mammals and the vegetation that comprised their foodstuffs. This isotope enrichment factor applies to a wide variety of ruminant mammals. Non-ruminant ungulates have a similar isotope enrichment, although our data cannot determine if it is significantly different. We also found a C isotope enrichment ?* of 3.1?±?0.7‰ for horn relative to diet, and 11.1?±?0.8‰ for enamel relative to horn for ruminant mammals. Tooth enamel is a faithful recorder of diet. Its isotopic composition can be used to track changes in the isotopic composition of the atmosphere, determine the fraction of C or C biomass in diets of modern or fossil mammals, distinguish between mammals using different subpathways of C photosynthesis,and identify those mammals whose diet is derived from closed-canopy habitats.
DOIPMID [本文引用: 2]

The diet of extant elephants (Loxodonta in Africa, Elephas in Asia) is dominated by C browse although some elephants have a significant C grass component in their diet. This is particularly noteworthy because high-crowned elephantid cheek teeth represent adaptation to an abrasive grazing diet and because isotopic analysis demonstrates that C vegetation was the dominant diet for Elephas in Asia from 5 to 1?Ma and for both Loxodonta and Elephas in Africa between 5-1?Ma. Other proboscideans in Africa and southern Asia, except deinotheres, also had a C-dominated diet from about 7?Ma (when the C biomass radiated in tropical and subtropical regions) until their subsequent extinction.
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 4]
DOIURL [本文引用: 1]
DOIURL
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 3]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
[本文引用: 1]
DOIURL [本文引用: 3]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

The hominin record from southern Asia for the early Late Pleistocene epoch is scarce. Well-dated and well-preserved fossils older than ~45,000 years that can be unequivocally attributed to Homo sapiens are lacking. Here we present evidence from the newly excavated Fuyan Cave in Daoxian (southern China). This site has provided 47 human teeth dated to more than 80,000 years old, and with an inferred maximum age of 120,000 years. The morphological and metric assessment of this sample supports its unequivocal assignment to H. sapiens. The Daoxian sample is more derived than any other anatomically modern humans, resembling middle-to-late Late Pleistocene specimens and even contemporary humans. Our study shows that fully modern morphologies were present in southern China 30,000-70,000 years earlier than in the Levant and Europe. Our data fill a chronological and geographical gap that is relevant for understanding when H. sapiens first appeared in southern Asia. The Daoxian teeth also support the hypothesis that during the same period, southern China was inhabited by more derived populations than central and northern China. This evidence is important for the study of dispersal routes of modern humans. Finally, our results are relevant to exploring the reasons for the relatively late entry of H. sapiens into Europe. Some studies have investigated how the competition with H. sapiens may have caused Neanderthals' extinction (see ref. 8 and references therein). Notably, although fully modern humans were already present in southern China at least as early as ~80,000 years ago, there is no evidence that they entered Europe before ~45,000 years ago. This could indicate that H. neanderthalensis was indeed an additional ecological barrier for modern humans, who could only enter Europe when the demise of Neanderthals had already started.
DOIURL [本文引用: 8]
[本文引用: 3]
[本文引用: 3]
DOIURL [本文引用: 5]
DOIURL [本文引用: 2]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 3]

Oxygen isotope analyses of skeletal remains (O-18/O-16, delta O-18) are a powerful tool for exploring major themes in bioarchaeology (the study of biological archaeological remains) and can aid in the reconstruction of past human environment interactions, socio-cultural decisions and individual life histories. Making use of the preserved animal and human tooth and bone commonly found at archaeological sites, applications include the reconstruction of palaeoclimate and palaeoseasonality; animal husbandry and management practices; human and animal lifetime mobility and provenance; and cultural practices such as breastfeeding, weaning and even past culinary preparation techniques. With a range of other uses across the natural, physical, chemical and biological sciences, oxygen isotope analyses are also highly cross-disciplinary, with developments in the field of isotope bioarchaeology potentially feeding into other fields and vice-versa. The purpose of this paper is to provide a summary of the biogeochemical background of oxygen isotope systematics from the water cycle to human and animal skeletal tissues for archaeologists and other scientists, and to explore how these have been utilised in terrestrial bioarchaeological research. In this way, we aim to provide an overview resource for stable isotope analysts in archaeology and the wider earth science community, as well as for archaeological practitioners and consumers interested in specific applications. By providing a summary of fundamental isotope mechanics alongside a review of recent developments in the field, we hope to highlight the potential of oxygen isotope bioarchaeology to not only reveal environmental and ecological aspects of the past relevant to human groups using archaeological materials, but also to illuminate past human decisions and behaviours. Current limitations and caveats of the approaches used are also explored.
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Thailand's geographical location in the tropics and almost complete, relatively uninterrupted forest cover makes it valuable for paleodiet and paleoclimate research. We present the first dietary and environmental reconstructions in Northeastern Thailand, using stable isotope abundances in mammalian tooth enamel from the late Middle Pleistocene locality, Tham Wiman Nakin (Snake Cave), which reflect a much higher (over 70%) than modern (13%) occurrence of C4 plants. Bovids and cervids appear to have had almost entirely a C4 plant diet. Carnivores consumed a mixture of C3 (suids) and C4 (bovids, cervids) consumers. Rhinoceroses and orangutan appear to have maintained their preference through time for forested or open C3 environment, respectively. (13)C/(12)C from bone bioapatite, horn and hair of modern Southeast Asian mammals almost exclusively demonstrate C3 vegetation dominance. C4 consumption is rare in analysed modern species and it could be related to anthropogenic influences such as ingestion of domestic crops or livestock. Interesting implications emerge in the C4 vegetation distribution in southern Eurasian ecosystems, indicating that Southeast Asia, south of the Tibet, could be part of the global C4 vegetation spread, which occurred around 7 Ma. However, the C4 percentage in ecosystems varied geographically. Despite modern reversal towards C3 habitats due to factors such as increasing CO(2), we think that anthropological influences may be responsible for habitat and dietary changes in extant species. Bovids demonstrate the most significant shift in diet and habitat through time, from C4-dominated open habitats to C3-dominated habitats indicative of dense forest understory.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 3]
DOIURL [本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 6]
PMID [本文引用: 1]

A new approach to proboscidean evolution depicts taxa in three major radiations. This approach highlights general proboscidean evolutionary trends and origins more than the specific relationships among them. Data from more than 55 million years of evolution help to interpret how the integration of primitive and derived characters was essential to proboscidean success. Only two, or perhaps three, species remain of approximately 164 that lived in the past. Extinct forms were extremely cosmopolitan, occupying a variety of habitats, from deserts to mountain tops, on all continents except Australia and Antarctica. Challenges for future investigators include a better understanding of structure and function of infrasonic call production and perception, brain features, and reproductive biology in extinct proboscideans based on inferences from living forms.
[本文引用: 4]
[本文引用: 3]
DOIURL [本文引用: 1]
[本文引用: 3]
DOIURL [本文引用: 2]
DOIPMID [本文引用: 1]

Stable carbon isotope ratios in bone collagen have been used in a variety of dietary studies in modern and fossil animals, including humans. Inherent in the stable isotope technique is the assumption that the isotopic signature is a reflection of the diet and is persistent in collagen because this is a relatively inert protein. Carbon isotope analyses of bones from a southern Indian population of Asian elephant (Elephas maximus), a long-lived mammal that alternates seasonally between a predominantly C (browse) and C (grass) plant diet, showed two patterns that have important implications for dietary interpretation based on isotopic studies. Relative to the quantity of the two plant types consumed on average, the δC signal in collagen indicated that more carbon was incorporated from C plants, possibly due to their higher protein contribution. There was a much greater variance in δC values of collagen in sub-adult (range -10.5‰ to-22.7‰, variance=14.51) compared to adult animals (range -16.0‰ to -20.3‰, variance=1.85) pointing to high collagen turnover rates and non-persistent isotopic signatures in younger, growing animals. It thus seems important to correct for any significant relative differences in nutritive value of food types and also consider the age of an animal before drawing definite conclusions about its diet from isotope ratios.
[本文引用: 2]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
PMID [本文引用: 2]

This paper investigates the utility of stable carbon and oxygen isotopes in human dental enamel to reveal patterns of breastfeeding and weaning in prehistory. Enamel preserves a record of childhood diet that can be studied in adult skeletons. Comparing different teeth, we used delta13C to document the introduction of solid foods to infant diets and delta18O to monitor the decline of breastfeeding. We report enamel carbonate delta13C and delta18O of 33 first molars, 35 premolars, and 25 third molars from 35 burials from Kaminaljuyú, an early state in the valley of Guatemala. The skeletons span from Middle Preclassic through Late Postclassic occupations, ca. 700 B.C. to 1500 A.D. Sections of enamel were removed from each tooth spanning from the cusp to the cemento-enamel junction. Stable isotope ratios were measured on CO2 liberated by reaction of enamel with H3PO4 in an automated carbonate system attached to a VG Optima mass spectrometer. Within a skeleton, teeth developing at older ages are more enriched in 13C and more depleted in 18O than teeth developing at younger ages. Premolars average 0.5/1000 [corrected] higher in delta13C than first molars from the same skeleton (P = 0.0001), but third molars are not significantly enriched over premolars. The shift from first molars to premolars may be due to the shift to solid foods from lipid-rich milk. After 2 years, when premolars begin to mineralize, the delta13C in childhood diets did not change systematically. First molars and premolars are similar in delta18O, but third molars average 0.7/1000 [corrected] lower than first molars (P = 0.0001) and 0.5/1000 [corrected] lower than premolars (P = 0.0003). First molar and premolar delta18O is heavier, because breast milk is more enriched in 18O than is drinking water. Hence, many children continued to nurse during the period of premolar formation. Together, these results indicate that Kaminaljuyú children had begun to eat solid maize foods before the age of 2 years but continued to drink breast milk until much later.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
