

1. College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China;
2. National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College & Testing Center, Fuwai Hospital, Beijing 100044, China
Received 17 December 2018; Revised 8 May 2019
Foundation items: Supported by the National Nature Science Foundation of China (31571481, 31771585)
Corresponding author: DING Yongsheng, E-mail: dingysh@ucas.edu.cn
Abstract: We describe a dispersive solid-phase ion exchange extraction method combined with capillary electrophoresis-laser induced fluorescence detection for the determination of asymmetric dimethylarginine, symmetric dimethylarginine, and monomethylarginine in serum samples. The methylated arginine-extracted resin beads were added into a mixed solution of 4-chloro-7-nitrobenzofuran acetonitrile solution and alkaline sodium borate solution (pH=10. 5) for the derivatization in a 60℃ water bath. The three derivatives were subjected to baseline separation in a fused silica capillary under the conditions of a separation electrolyte of 80 mmol/L phosphate (pH=2. 0) and a separation voltage of 20 kV. On the basis of the FDA bioanalytical method validation guidance, the method validation was conducted and the results were satisfactory. This method was used to determine the three methylated arginines in the serum samples from local hospital.
Keywords: methylated argininesdispersive solid-phase extraction4-chloro-7-nitrobenzofurazancapillary electrophoresis-laser induced fluorescence
分散固相萃取结合毛细管电泳-激光诱导荧光法测定血清中甲基化精氨酸
谢士杰1, 杨泽诚1, 周洲2, 蔺亚辉2, 苏保满2, 丁永胜1


1. 中国科学院大学生命科学学院, 北京 100049;
2. 中国医学科学院/北京协和医学院 国家心血管病研究中心, 北京阜外医院检验中心, 北京 100044
摘要: 介绍一种分散固相离子交换萃取方法,结合毛细管电泳-激光诱导荧光检测法,测定血清样品中不对称二甲基精氨酸、对称二甲基精氨酸和单甲基精氨酸。将提取甲基化精氨酸的树脂粒加入到4-氯-7-硝基苯并呋喃乙腈溶液和碱性硼酸钠溶液(pH=10.5)的混合溶液中,于60℃水浴中衍生化。在80 mmol/L磷酸盐溶液(pH=2.0)分离电解质和20 kV分离电压条件下,这3种衍生物在熔融石英毛细管中获得基线分离。根据FDA生物分析方法验证指南,进行方法验证,结果满意。利用该方法测定了当地医院血清样品中的3种甲基化精氨酸含量。
关键词: 甲基化精氨酸分散固相萃取4-氯-7-硝基苯并呋喃毛细管电泳-激光诱导荧光
Three endogenous methylated arginine analogies, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and monomethylarginine (MMA) are generated by the degradation of proteins containing methylated arginine residues. ADMA is considered to be an endogenous inhibitor of nitric oxide production and is associated with many diseases[1-4], but SDMA and MMA are relatively less concerned. Recently, arginine methylation index, which was composed of the sum of the dimethylated species concentrations divided by the concentration of the monomethylated form, was suggested to predict the extent of disease progression[5].
Currently, chromatography coupled with mass spectrometry (MS) and enzyme-linked immunosorbent assay (ELISA) are widely used for the determination of these substances in biological samples[6-16]. However, there are a few of disadvantages such as high cost of MS and single target testing of ELISA. Capillary electrophoresis (CE) with high separation efficiency and low reagent consumption is an alternative method for the determination of ADMA in biological sample coupled with ultraviolet detection or laser-induced fluorescence (LIF) detection[17-18]. Because of below μmol/L level of the methylated arginines and complex matrix interference in the blood, various sample preparation techniques have been applied to concentrate analyte and eliminate interference, such as field amplification sample injection[19], solid phase extraction[20], and ultrafiltration[21].
Dispersive solid phase extraction (DSPE) is an easy-to-do technique. It consists of two steps, dispersion of the acceptor phase (solid sorbent) into a donor phase (sample solution) and phase separation by centrifugation or magnetic force. The approach enables the sorbent to interact equally with the whole sample to reach great capacity per amount of sorbent. Thus, DSPE has been considered as a quick, easy, cheap, effective, rugged, and safe approach for a wide scope of sample preparation. Considering the convenience and practicality, three beads (about 1 mm diameter) of a strong cation exchange resin were selected as the sorbent to extract these positively charged methylated arginines from dozens of microliters of serum sample. The separation between the solid phase and the liquid phase was carried out by taking out the resin beads or aspirating the liquid without any equipment. Based on this DSPE and 4-chloro-7-nitrobenzofuran (NBD-Cl) derivatization, a CE-LIF method was developed for the determination of ADMA, SDMA, and MMA in human serum samples.
1 Materials and methods1.1 Chemicals and solutionsADMA, SDMA, MMA, NBD-Cl, sodium dihydrogen phosphate, and sodium borate were purchased from Sigma-Aldrich (Shanghai, China). L-arginine was purchased from Sigma-Aldrich (Dr. Ehrenstorfer, Germany). Anhydrous ACN was of analytical grade and obtained from Sinopharm Chemical Reagent (Shanghai, China). Cation exchange resin was purchased from Nankai University Resin Co., Ltd (Tianjin, China). Each of the MMA, ADMA, and SDMA standards was dissolved directly in deionized water to prepare the stock solutions. These stock solutions were aliquoted and stored at -20 ℃ until use and then diluted with the deionized water to the desired concentration. NBD-Cl was dissolved in anhydrous ACN to form a concentration of 200 mmol/L. All stock solutions were kept in the dark at -20 ℃ for storage. The pH of solution was adjusted with 1 mol/L HCl or 1 mol/L NaOH by a digital pH meter (Denver Instrument, Denver, UT, USA).
1.2 DSPE sample preparationThe human serum samples were collected from a cardiovascular specialist hospital and a general hospital. All serum samples were stored at -20 ℃ until use. The sample preparation is described as follows: 1) mixing serum sample with acetonitrile at a volume ratio of 1:1 and removing the precipitate by a centrifugation at 12 000 rpm for 5 min, 2) adding three beads of the cation exchange resins (H+ form, about 1 mm diameter) in a 1.5 mL plastic tube followed by injecting 80 μL deproteinized serum sample and shaking the mixture for 3 min to promote the exchange of the cations, 3) separating the exchanged beads from the solution and rinsing the beads three times with deionized water, 4) mixing the washed beads with 10 μL of 100 mmol/L NBD-Cl and 70 μL of 10 mmol/L borate (pH=10.5) for the derivatization, and 5) conducting the reaction in 60 ℃ water bath for 30 min. The above procedures of the entire sample preparation are shown in Fig. 1.
Fig. 1
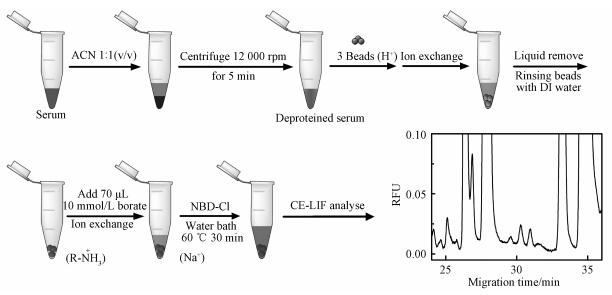 | Download: JPG larger image |
Fig. 1 Description of the DSPE sample preparation | |
1.3 Apparatus and proceduresA Beckman-Coulter P/ACE MDQ (Fullerton, CA, USA) CE instrument was equipped with a LIF detector and an argon ion laser. The excitation and emission wavelengths were 488 and 520 nm, respectively. Electrophoresis was performed in a 60-cm fused-silica capillary (Yongnian, Baoding, Hebei Province, China) with 50-cm effective length (75 μm i.d. and 375 μm o.d.), with the anode and cathode positioned at the inlet and outlet ends of the capillary, respectively. Data collection, processing, and analysis were performed using a system 32-Karat software 8.0 (Beckman) and recorded on a personal computer. The capillary was treated prior to its first use by sequentially flushing with methanol for 20 min/138 kPa, 1.0 mol/L NaOH for 20 min/138 kPa, deionized water for 10 min/138 kPa, and running buffer for 10 min/138 kPa. At the end of each day, the capillary was rinsed sequentially with methanol (5 min/138 kPa), 0.1 mol/L NaOH (5 min/138 kPa), deionized water (5 min/138 kPa), and air-dried (5 min/138 kPa). The running buffer (80 mmol/L phosphate, pH=2.00) was degassed by sonication for 10 min before use. Unless noted, the sample solution was introduced into the capillary by a hydrodynamic injection (3.4 kPa pressure injection for 5 s) and subsequently separated with an applied potential of 20 kV and a controlled temperature of 20 ℃. The injection volume was about 24.7 nL and calculated by the equation V=ΔPd4πt/128ηL. (ΔP is the pressure difference between the ends of the capillary, d is the inner diameter of the capillary, t is the injection time, η is the sample viscosity, and L is the total length of the capillary). Although these procedures improved day-to-day and capillary-to-capillary reproducibility, the slight shifts in migration time were still unavoidable. Thus each series of experiments was performed under the same day and capillary as possible.
2 Results and discussion2.1 DSPEAs a simple sample preparation technique, DSPE is highly efficient and environmentally friendly. In this study, only three beads (about 1 mm diameter) of the strong cation exchange resin were selected as the solid phase for the sample preparation. The extraction efficiency depends on the exchange between the hydrogen ions on the resin and the positively charged analytes in the solution. The average exchange capacity of each bead can be calculated on the basis of the change in the pH of 0.1 mol/L sodium chloride before and after adding the hydrogen-form resin beads into the salt solution, where the exchange between sodium ions in the solution and hydrogen ions on the resin occurs. The average exchange capacity of each bead was calculated to be about 0.9 μmeq. The concentration of analyte in sample can be calculated by the amount of analyte retained on solid phase and the volume of sample. Take into account the reduction in the amount of serum and derivatization reagents, three beads of the resin with about 2.7 μmeq of the exchange capacity was selected to extract the analytes form the deproteined serum sample. Figure 2(a) shows that the peak areas of the analytes in 20 to 80 μL of DSPE-treated serum samples are proportional to the serum volume under the same derivatization condition. In addition, when the volume of the serum sample exceeds 80 μL, the amount of the analytes extracted by three resin beads is close to saturation. However, if the resin number is increased, a larger volume of serum sample can be processed, resulting in a larger concentration factor. Figure 2(b) is a comparison of electrophoretic separation of 20 μL serum samples without DSPE and 20 and 80 μL serum samples treated with DSPE, in which the peak areas of the analytes are significantly multiplied in the DSPE-treated 80 μL serum sample.
Fig. 2
 | Download: JPG larger image |
Separation conditions: background electrolyte=80 mmol/L phosphate (pH=2.0); sample injection=0.5 psi for 5 s; separation voltage=20 kV; cartridge temperature=20 ℃. Peak names: 1=MMA, 2=ADMA, 3=SDMA. Ⅰ: 20 μL serum sample without DSPE, Ⅱ and Ⅲ: 20 and 80 μL serum samples by DSPE, respectively in (b). Fig. 2 Relationship between serum sample volume and DSPE extraction efficiency | |
2.2 NBD-Cl derivatizationNBD-Cl is non-fluorescent and can react with amino group-containing compounds, such as aliphatic amines and amino acids, to form highly fluorescent compounds. The maximum excitation and emission wavelength of the arginine-NBD product in an aqueous solution is 470 and 560 nm, respectively. This property partially matches the excitation wavelength (488 nm) and emission wavelength (520 nm) of the argon fluorescence detector used in this experiment. Since the reaction between NBD-Cl and amino acid is a nucleophilic substitution reaction, the increase in the pH of the reaction medium can favorite the formation of a positively charged carbon atom by the leave of the chlorine ion of NBD-Cl and the further formation of a new C-N bond with amino group of amino acid. Thus, the effect of the pH of the reaction medium (10 mmol/L borate) between 7.5 and 11.5 on the peak area of arginine-NBD derivatives in the standard solution was investigated. Figure 3(a) shows that all peak areas of the derivatives reach a maximum at the pH of 8.5. However, the dispersive resin can decrease the pH of the reaction medium due to the released hydrogen ions from the resin. Since the total volume of the entire derivatization reaction is less than 100 μL, the change in the pH of the reaction medium was investigated by mixing three extracted beads with the borate solutions at the different pH. The pH value was estimated using a test paper scale. As shown in Fig. 3(b), when the pH of the borate solution was above 10.5, the pH of the solution with exchanged beads reached over 8. The electropherogram in Fig. 3(c) showed that the peaks of these analytes were not visible when the pH of the borate solution was below 9.5, whereas the target peaks appeared when the pH reached above 10.5 and these peaks at 11.5 were smaller than those at 10.5. This phenomenon indicated that the analytes on the resin were released into the solution and then subjected to a derivatization reaction at a suitable alkaline pH condition. Considering the presence of a large amount of other amino acids in serum samples, deproteinized serum samples were used directly for derivatization to optimize NBD-Cl concentrations to ensure that a sufficient amount of NBD-Cl was involved in the derivatization. As shown in Fig. 3(d), when the concentration of NBD-Cl was increased from 50 to 100 mmol/L, the peak area of the analytes continued to increase, while the peak area did not change significantly between 100 and 150 mmol/L. Therefore, the concentration of NBD-Cl was selected to be 100 mmol/L. In addition, the temperature and time of the derivatization reaction were optimized at 60 ℃ and 30 min, respectively.
Fig. 3
 | Download: JPG larger image |
All experiments were performed under the optimal conditions unless otherwise indicated.Peak names:1=MMA, 2=ADMA, 3=SDMA. Fig. 3 Optimization of the derivatization conditions | |
2.3 Electrophoretic separationAmino acid-NBD derivatives can be protonated under the acidic separation buffer condition. In the case of acidic conditions (pH less than 3), the electroosmotic flow fused-silica capillary is close to zero, the separation of the analytes will depend on the difference in charge and molecular weight. Considering the presence of a large number of amino acids in serum samples, we chose arginine, histidine and glycine mixed with three methylated arginine to optimize the separation conditions. The pH of the separation buffer is a key factor in the separation of three analytes. As shown in Fig. 4(a), when the pH was lower than 2.0, the histidine peak emerged with the MMA peak (pH=1.8) and the SDMA peak (pH=1.9); on the other hand, when the pH was higher than 2.0, the resolution between the ADMA and SDMA peaks was 0.63 (pH=2.1) and 0.49 (pH=2.2). Finally, the six analytes were completely separated at the pH of 2.0.
Fig. 4
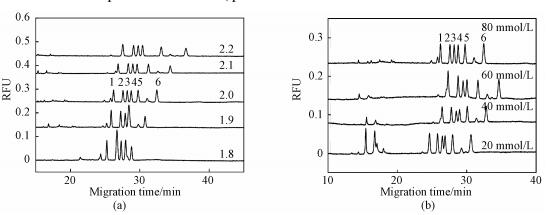 | Download: JPG larger image |
Peak names: 1=Arginine, 2=MMA, 3=ADMA, 4=SDMA, 5=Histidine, 6=Glycine. All experiments were performed under the optimized condition unless otherwise indicated. Fig. 4 Effects of the pH (a) and the concentration (b) of phosphate buffer on the separation of the analytes | |
The increase in concentration of background electrolyte generally results in a decrease in electroosmotic flow due to reduction of zeta potential. However, in this experiment, the electroosmotic flow was almost equal to zero and a large amount of anions that migrated in the opposite direction caused drag on the forward-migrating analyte cation. As shown in Fig. 4(b), when the phosphate concentration was increased from 20 to 80 mmol/L, the resolution between ADMA and SDMA was increased from 0.52 to 1.12. Although the phosphate concentration showed a certain influence on the separation of the analyte, high concentration of background electrolyte easily caused bubbles. Therefore, the phosphate concentration was chosen to be 80 mmol/L.
2.4 Method validationPrior to the real sample analysis, the method was verified according to a FDA guideline (Guidance for Industry: Bioanalytical Method Validation). The repeatability was evaluated by measuring the intra- and interday precisions in terms of the peak area and migration time at three different concentrations of the added standard (0.25, 0.5, and 1.0 μmol/L). Briefly, the intraday RSDs of peak area and migration time were less than 3.64% and 1.02%, respectively. The interday RSDs of peak area and migration time were less than 4.83% and 1.32%, respectively. The linearity was evaluated by the addition of five level of the standards (0.05 to 5.0 μmol/L) into the serum sample with the correlation coefficient larger than 0.99. According to the signal-to-noise ratio larger than 3, the limits of detection of ADMA, SDMA and MMA were 14.9, 12.2 and 17.5 nmol/L, respectively. The recovery test was performed by the addition of three levels of the standards into the serum samples to compare the measured amount of the analytes with the actual amounts of the added analytes to the samples. The details of the results of the method validation are summarized in Table 1.
Table 1
| Table 1 Results of the method validation | ||||||||||||||||||||||||||||||||||||||||||||||||||||
2.5 Real sample analysisSince human serum originally contains the methylated arginines, standard addition quantification can eliminate the effects of sample matrix. Based on a plot between the responses of analytes and the concentration of the added standards, the concentration of analyte in the original sample was obtained by extrapolating the plot to zero response. Using the above developed method, the contents of ADMA, SDMA, and MMA in serum samples from outpatients and inpatients were evaluated. The outpatient group was 20 serum samples from unclassified visitors from a local general hospital. The inpatient group was 20 serum samples from hospitalized patients with various cardiovascular diseases from a local cardiovascular specialist hospital. The results of the real sample analysis are shown in Fig. 5. Overall, the mean ADMA level in the inpatient group is significantly higher than that in the outpatient group, whereas the mean SDMA and MMA levels were not significantly different between the outpatient and inpatient groups. However, there is a more pronounced difference in the mean arginine methylation index between inpatients and outpatient groups. This suggests that arginine methylation index might be more suitable for clinical evaluation than ADMA concentration. According to the above statistical analysis, the higher ADMA concentration and arginine methylation index in serum are more likely to occur in patients with cardiovascular disease than in other general patients. However, at the individual level, the association of sample ADMA concentrations with cardiovascular disease cannot be determined. Therefore, further assessment of the association between methylated arginine and cardiovascular disease may require more clinically traceable subjects.
Fig. 5
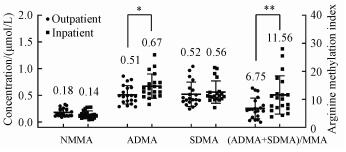 | Download: JPG larger image |
Statistical differences between the outpatient and inpatient groups were evaluated by one-way ANOVA student′s t-test (*p < 0.05, **p < 0.01). Fig. 5 Results of the real sample analyses | |
3 ConclusionsA simple and inexpensive DSPE sample preparation combined with CE-LIF to determine three methylated arginines in human serum was developed. Compared with other solid phase extraction techniques, the DSPE here uses only three cation exchange resin beads to conduct methylated arginine extraction in a small volume of serum sample, which exhibits the outstanding advantages in concentrating targets and eliminating interference. This analytical method will facilitate the investigation of the association between the level of three methylated arginines (e.g. arginine methylation index) in the blood and the related diseases.
References
| [1] | Bouras G, Deftereos S, Tousoulis D, et al. Asymmetric dimethylarginine (ADMA):a promising biomarker for cardiovascular disease?[J]. Current Topics in Medicinal Chemistry, 2013, 13: 180-200. |
| [2] | Kumarasamy C, Singh G, Raman P, et al. Effect of protein arginine methyltransferase-1 inhibition on hypoxia-induced vasoconstriction[J]. Medical Hypotheses, 2015, 85: 740-743. |
| [3] | Bajaj J S, Ahluwalia V, Wade J B, et al. Asymmetric dimethylarginine is strongly associated with cognitive dysfunction and brain MR spectroscopic abnormalities in cirrhosis[J]. Journal of Hepatology, 2013, 58: 38-44. |
| [4] | Inan B, Ates I, Ozkayar N, et al. Are increased oxidative stress and asymmetric dimethylarginine levels associated with masked hypertension?[J]. Clinical and Experimental Hypertension, 2016, 38: 294-298. |
| [5] | Wang Z, Tang W H, Cho L, et al. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2009, 29: 1383-1391. |
| [6] | Wi?niewski J, Fleszar M G, Piechowicz J, et al. A novel mass spectrometry-based method for simultaneous determination of asymmetric and symmetric dimethylarginine, L-arginine and L-citrulline optimized for LC-MS-TOF and LC-MS/MS[J]. Biomedical Chromatography, 2017, 31: e3994. |
| [7] | Kayacelebi A A, Kn?fel A K, Beckmann B, et al. Measurement of unlabeled and stable isotope-labeled homoarginine, arginine and their metabolites in biological samples by GC-MS and GC-MS/MS[J]. Amino Acids, 2015, 47: 2023-2034. |
| [8] | Martens-Lobenhoffer J, Rodionov R N, Bode-B?ger S M, et al. Determination of asymmetric Nα-acetyldimethylarginine in humans:a phase II metabolite of asymmetric dimethylarginine[J]. Analyical Biochemistry, 2014, 452: 25-30. |
| [9] | Kleparnik M, Tomandlova M, Glatz Z, et al. Determination of asymmetric and symmetric dimethylarginines in human plasma by HPLC with electrochemical detection[J]. Journal of Separation Science, 2013, 36: 3696-3701. |
| [10] | Davids M, Swieringa E, Palm F, et al. Simultaneous determination of asymmetric and symmetric dimethylarginine, L-monomethylarginine, L-arginine, andL-homoarginine in biological samples using stable isotope dilution liquid chromatography tandem mass spectrometry[J]. Journal of Chromatography B, 2012, 900: 38-47. |
| [11] | Shin S, Fung S M, Mohan S, et al. Simultaneous bioanalysis ofL-arginine, L-citrulline, and dimethylarginines by LC-MS/MS[J]. Journal of Chromatography B, 2011, 879: 467-474. |
| [12] | Gervasoni J, Bonelli F, Zuppi C, et al. Determination of asymmetric dimethyl arginine in human serum by liquid chromatography-tandem mass spectrometry:clinical application in hypertensive subjects[J]. Clinical Chemistry and Laboratory Medicine, 2011, 49: 2109-2115. |
| [13] | Brown C M, Becker J O, Wise P M, et al. Simultaneous determination of 6L-Arginine metabolites in human and mouse plasma by using hydrophilic-interaction chromatography and electrospray tandem massspectrometry[J]. Clinical Chemistry, 2011, 57: 701-709. |
| [14] | Gangi I M D, Chiandetti L, Gucciardi A, et al. Simultaneous quantitative determination of NG, NG-dimethyl-l-arginine or asymmetric dimethylarginine and related pathway's metabolites in biological fluids by ultrahigh-performance liquid chromatography/electrospray ionization-tandem mass spectrometry[J]. Analytica Chimica Acta, 2010, 677: 140-148. |
| [15] | Schwedhelm E, Maas R, Tan-Andresen J, et al. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma[J]. Journal of Chromatography B, 2007, 851: 211-219. |
| [16] | Yokoro M, Suzuki M, Yatani M, et al. Development of an enzyme-linked immunosorbent assay system for the determination of asymmetric dimethylarginine using a specific monoclonal antibody[J]. Bioscience Biotechnology and Biochemistry, 2012, 76: 400-403. |
| [17] | Zinellu A, Sotgia S, Zinellu E, et al. High-throughput CZE-UV determination of arginine and dimethylated arginines in human plasma[J]. Electrophoresis, 2007, 28: 1942-1948. |
| [18] | Linz T H, Snyder C M, Lunte S M. Optimization of the separation of NDA-derivatized methylarginines by capillary and microchip electrophoresis[J]. Journal of Laboratory Automation, 2012, 17: 24-31. |
| [19] | Zinellu A, Sotgia S, Usai M F, et al. Improved method for plasma ADMA, SDMA, and arginine quantification by field-amplified sample injection capillary electrophoresis UV detection[J]. Analytical and Bioanalytical Chemistry, 2011, 399: 1815-1821. |
| [20] | Teerlink T, Nijveldt R J, de Jong S, et al. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography[J]. Analytical Biochemistry, 2002, 303: 131-137. |
| [21] | Naz S, Garcia A, Rusak M, et al. Method development and validation for rat serum fingerprinting with CE-MS:application to ventilator-induced-lung-injury study[J]. Analytical and Bioanalytical Chemistry, 2013, 405: 4849-4858. |
