

中国科学院大学化学与化学工程学院, 北京 100049
摘要: 研究在短时间模拟太阳光照射或黑暗条件下,标准天然有机质Suwannee River腐殖酸将三价金离子(Au3+)还原成金纳米颗粒(AuNPs)的事实.研究结果表明,活泼的腐殖酸对还原金离子这一反应在水体中广泛存在,在极端缺乏光照的水体(如地下水、溶洞水等水域)中也可进行.Pony Lake富里酸和Aldrich腐殖酸的对比试验表明,不同天然有机质在缺乏光照条件下还原Au3+的能力有差异.
With the rapid development of nanotechnology in recent decades, industrial scale engineered nanop-articles (ENPs) are produced every year, including non-metals, carbon, polymers, lipids, metal oxides and metals[1]. ENPs are used in various fields such as energy, medical treatment, food products, electronics, and communications. Among them, gold nanoparticles (AuNPs) is one of the most common noble metal-containing nanoparticles (NPs)[2], whose applications have covered cellular imaging, bio-chemical sensing, drug, and medical therapeutics[3]. The expanding applications and production of AuNPs inevitably lead to its accumulating release and exposure in surface environment, which has raised wide concern about environmental toxicity of AuNPs. In the aquatic ecosystem, AuNPs can be taken up by organisms, which then causes toxicity and adhesion[4-6], as well as cycling through the food chain.
NOM is a complex mixture of poorly understood organic molecules. NOM exsits in abundance in natural waters, and the concentration can be up to 15mg/L[7]. Containing carbonyl-, carboxyl-, phenol-, nitrogen-, and sulfur-containing groups, NOM has high affinities for metal ions[8]. Recent reports has suggested that natural organic matter (NOM) reduces Au(III) to form AuNPs in the aquatic environment under continuous irradiation[9-10]. Additionally, NOM influences fate of NPs and transport NPs by stabili-zing and dispersing NPs as a coating agent[11-12].
Gold concentration can be up to about 80μg·L-1 in ground water[13-16]. Recently, the stable existence of Au(III)-complexes in aqueous environment has also been reported[17]. So it is necessary to further expand our knowledge of the aqueous production of AuNPs from Au(III) complexes by NOM in aquatic systems. Existing studies[18-21] have focused on the transformation of ionic metals under continuous irradiation in systems with NOM. However, to our best knowledge, research on the photoreduction of Au3+ by different NOMs with limited irradiation exposure has not been reported. In this work, the specific behaviors of Au3+ with different NOMs in aqueous environment undergoing full-course darkness or simulated sunlight of short durations are examined.
1 Experimental section1.1 MaterialsGold(III) chloride hydrate (>99.9% purity) was from J&K. Suwannee River humic acid (SRHA) and Pony Lake fulvic acid (PLFA) were from the International Humic Substances Society (IHSS). Aldrich humic acid (AHA) was obtained from Aldrich. Sodium borohydride was obtained from Alfa Aesar. Water was obtained from a Milli-Q purification system. Total organic carbon (TOC) of the NOM solutions was analyzed by a Tekmar-Dohrmann Apollo 9000 TOC analyzer.
1.2 Reaction proceduresAll the experiments were performed in quartz beakers, and the total volume of each reaction solution was 20mL. Solution conditions were 0.2mmol/L HAuCl4 and 10mg C/L (carbon content per litre) NOM (SRHA, PLFA or AHA). The control samples in the presence of 0.2mmol/L HAuCl4 and 10mg C/L NOM were exposed to simulated sunlight for 5-30min and then kept in dark. Simulated sunlight was provided by a 250W Xe lamp.
1.3 AnalysisAuNPs were completely removed from the solutions by centrifuging the samples at 16000rpm for 15min. Then, 1mL supernatant was taken out and diluted five times with water for measuring the remaining Au3+ concentration. The Au3+ concentration determination was performed via the flame method using an atomic absorption spectrometer (PinAAcle 900T, PerkinElmer, USA). Temporal reduction ratios of Au3+ were calculated according to the fomula, [AuNP]t/[Au3+]0=([Au3+]0-[Au3+]t)/[Au3+]0. [Au3+]0 is the Au3+ concentration at time t=0; [Au3+]t is the Au3+ concentration at time t=t; and [AuNP]t is the AuNPs concentration at time t=t.
UV-Vis spectra were recorded from 200 to 800nm by a TU-1900 spectrophotometer (Beijing Purkinje General Instrument Co. Ltd.). Transmission electron microscopy (TEM) was performed using a Tecnai G2 F30 instrument (FEI, USA).
2 Results and discussion2.1 Reduction of Au3+ by SRHA after short-term simulated solar irradiationThe formation of AuNPs in the presence of 10mg C/L SRHA and 0.2mmol/L HAuCl4 in darkness is shown in TEM picture (Fig. 1). The shape of AuNPs are mainly sphere, flake, and rod-like structures.
Fig. 1
 | Download: JPG larger image |
Solution conditions: 0.2mmol/L HAuCl4 and 10mg C/L SRHA. Fig. 1 TEM images of gold nanoparticles formed in darkness | |
For SRHA, first, the temporal yields of AuNPs in the full-course darkness showed little difference compared with those underwent 10-min irradiation, which reveals the considerable activity of SRHA to reduce Au3+ in the dark (Fig. 2). Secondly, after short-term irradiation, the yields of AuNPs continued to increase in the dark. After six-day photophobic placement, ~80% of the Au3+ ions in the samples were reduced to form AuNPs. Moreover, the longer the irradiation continued in the first step, the closer to 100% the final AuNPs formation ratio in the sample was. It is suggested that irradiation enhances the reaction rate, which is attributed to the O2·- generation under simulated sunlight[9].
Fig. 2
 | Download: JPG larger image |
Solution conditions: 0.2mmo/L HAuCl4 and 10mg C/L SRHA. Fig. 2 Temporal yields of AuNPs in the dark after different durations of irradiation | |
Besides, temporal reduction ratios of Au3+ by SRHA under continuous irradiation reached 86.8% in 1h and almost 100% in 2h (Table 1). These results illustrate that both short-term irradiation and dark condition led to significant decrease of AuNPs formation rate, compared with continuous irradiation process.
Table 1
| Table 1 Temporal reduction ratios of Au3+ under continuous simulated sunlight | ||||||||||||||||||||||||
For the full-course darkness experiments, it should be taken into consideration that the absolute darkness was unreached in the lab. Once the superoxide free radical produced before the samples were placed into darkness, it may partly cause the reduction of Au3+. Gold atoms nucleate within minutes or even seconds of irradiation, and once the concentration of AuNPs increases, the likelihood of AuNPs to fuse, grow, or aggregate increases.
Under some circumstances, free ionic Au3+ and other metal ions in the aquatic environment are exposed to sunlight for a short period and then remain in the dark or shadow. For example, in a substantial part of the Earth surface such as deepwater areas, there is only short-term exposure or even no exposure to sunlight. In addition, the complexity of the ground-surface conditions should be taken into consideration, for example, underground streams and cavern water. Hence, the above results imply that, even in aquatic environments with low sunlight exposure, reduction of Au3+ by active NOM like SRHA can still occur.
2.2 Analogous experiments using AHA and PLFAFor PLFA and AHA, the processes of short-time irradiation and full-course darkness were studied (Fig. 3). In full-course darkness or after short irradiation, the reduction ratios of Au3+ were much lower than under the lasting simulated sunlight irradiation (Table 1) for both PLFA and AHA, which is consistent with the results of SRHA. For both PLFA and AHA, we found small difference in the results in full-course darkness or after 10-to 30min irradiation. It is further demonstrated that these samples are obviously less active than SRHA.
Fig. 3
 | Download: JPG larger image |
Solution conditions: 0.2mmol/L HAuCl4 and 10mg C/L PLFA (a) or AHA (b). Fig. 3 Temporal yields of AuNPs in the dark after different durations of irradiation by NOMs | |
As shown in Fig. 4, in electron paramagnetic resonance (EPR) spectra of PLFA there was no significant signal of superoxide species in darkness (0min). However, after simulated sunlight irradiation, the superoxide species content increased evidently with time. This clearly proves the effect of O2 in the Au3+ reduction by NOMs and explains why NOM samples under lasting exposure reduce Au3+ to gold nanoparticles fast.
Fig. 4
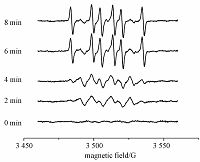 | Download: JPG larger image |
Initial concentrations: 100mg C/L PLFA; 40mmol/L DMPO. Fig. 4 Corresponding DMPOO2·- EPR spectra of PLFA after different durations of simulated sunlight irradiation | |
These results suggest that not all NOMs can operate non-photochemical processes. The active NOM like SRHA could reduce metal ions in dim environment. But NOMs like PLFA, whose aromatic and carbonyl contents are much lower, cannot reduce Au3+ in the dark unless with long term photoexcitation. The specific reasons may be complex and related to some physical and chemical properties of different samples, which needs further studies in the future.
3 ConclusionIn brief, this study covers the Au3+ photoreduction by three kinds of NOMs under different irradiation conditions, including full-course darkness and short-term and continuous simulated sunlight irradiation. It provides valuable information about different reaction process in the complex surface water environment. The almost complete reduction of Au3+ to AuNPs by SRHA in the dark after short-term irradiation suggests that the reduction of metal ions by active NOM may occur widely, even in special aquatic environments that lack sunlight exposure such as underground and cavern water.
References
| [1] | Nowack B, Bucheli T D. Occurrence, behavior and effects of nanoparticles in the environment[J].Environmental Pollution, 2007, 150:5–22.DOI:10.1016/j.envpol.2007.06.006 |
| [2] | Weinberg H, Galyean A, Leopold M. Evaluating engineered nanoparticles in natural waters[J].Trends in Analytical Chemistry, 2011, 30:72–83.DOI:10.1016/j.trac.2010.09.006 |
| [3] | Bozich J S, Lohse E, Torelli M D, et al. Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna[J].Environmental Science:Nano, 2014, 1:260–270.DOI:10.1039/C4EN00006D |
| [4] | Skjolding L M, Hjorth R, Hartmann N, et al. Uptake and depuration of gold nanoparticles in Daphnia magna[J].Ecotoxicology, 2014, 23:1172–1183.DOI:10.1007/s10646-014-1259-x |
| [5] | Garcia-Cambero J P, Nunez Garcia M, Lopez G D, et al. Converging hazard assessment of gold nanoparticles to aquatic organisms[J].Chemosphere, 2013, 9:1194–1200. |
| [6] | Lapresta-Fernandez A, Fernandez A, Blasco J. Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms[J].Trends in Analytical Chemistry, 2012, 32:40–59.DOI:10.1016/j.trac.2011.09.007 |
| [7] | Al-Reasi H A, Wood C M, Smith D S. Physicochemical and spectroscopic properties of natural organic matter from various sources and implications for ameliorative effects on metal toxicity to aquatic biota[J].Aquatic Toxicology, 2011, 103:179–190.DOI:10.1016/j.aquatox.2011.02.015 |
| [8] | Elbishlawi H, Jaffe P R. Characterization of dissolved organic matter from a restored urban marsh and its role in the mobilization of trace metals[J].Chemosphere, 2015, 127:144–151.DOI:10.1016/j.chemosphere.2014.12.080 |
| [9] | Yin Y, Liu J, Jiang G. Sunlight-induced reduction of ionic Ag and Au to metallic nanoparticles by dissolved organic matter[J].ACS Nano, 2012, 6:7910–7919.DOI:10.1021/nn302293r |
| [10] | Yin Y, Yu S, Liu J, et al. Thermal and photoinduced reduction of ionic Au(III) to elemental au nanoparticles by dissolved organic matter in water:possible source of naturally occurring au nanoparticles[J].Environmental Science and Technology, 2014, 48:2671–2679.DOI:10.1021/es404195r |
| [11] | Gao J, Powers K, Wang Y, et al. Influence of Suwannee River humic acid on particle properties and toxicity of silver nanoparticles[J].Chemosphere, 2012, 89:96–101.DOI:10.1016/j.chemosphere.2012.04.024 |
| [12] | Delay M, Dolt T, Woellhaf A, et al. Interactions and stability of silver nanoparticles in the aqueous phase:influence of natural organic matter (NOM) and ionic strength[J].Journal of Chromatography A, 2011, 1218:4206–4212.DOI:10.1016/j.chroma.2011.02.074 |
| [13] | Bowell R J, Foster R P. The mobility of gold in tropical rain forest soils[J].Economic Geology, 1993, 88:999–1016.DOI:10.2113/gsecongeo.88.5.999 |
| [14] | Reith F, Lengke M F, Falconer D, et al. The geomicrobiology of gold[J].ISME Journal, 2007, 1:567–584.DOI:10.1038/ismej.2007.75 |
| [15] | Williams-Jones A E, Bowell R J, Migdisov A. Gold in solution[J].Elements, 2009, 5(5):281–287.DOI:10.2113/gselements.5.5.281 |
| [16] | Usher A, McPhail DC, Brugger J. A spectrophotometric study of aqueous Au(III) halide-hydroxide complexes at 25-80℃[J].Geochimica et Cosmochimica Acta, 2009, 73(11):3359–3380.DOI:10.1016/j.gca.2009.01.036 |
| [17] | Ta C, Reith F, Brugger J, et al. Analysis of gold(I/III)-complexes by HPLC-ICP-MS demonstrates gold(III) stability in surface waters[J].Environmental Science and Technology, 2014, 48:5737–5744.DOI:10.1021/es404919a |
| [18] | Adegboyega N F, Sharma V K, Siskova K, et al. Interactions of aqueous Ag+ with fulvic acids:mechanisms of silver nanoparticle formation and investigation of stability[J].Environmental Science and Technoloy, 2013, 47:757–764.DOI:10.1021/es302305f |
| [19] | Hou W C, Stuart B, Howes R, et al. Sunlight-driven reduction of silver ions by natural organic matter:formation and transformation of silver nanoparticles[J].Environmental Science and Technoloy, 2013, 47:7713–7721.DOI:10.1021/es400802w |
| [20] | Yin Y, Shen M, Zhou X, et al. Photoreduction and stabilization capability of molecular weight fractionated natural organic matter in transformation of silver ion to metallic nanoparticle[J].Environmental Science and Technoloy, 2014, 48:9366–9373.DOI:10.1021/es502025e |
| [21] | Pham A N, Rose A L, Waite T D. Kinetics of Cu(II) reduction by natural organic matter[J].The Journal of Physical Chemistry A, 2012, 116(25):6590–6599.DOI:10.1021/jp300995h |
