摘要/Abstract
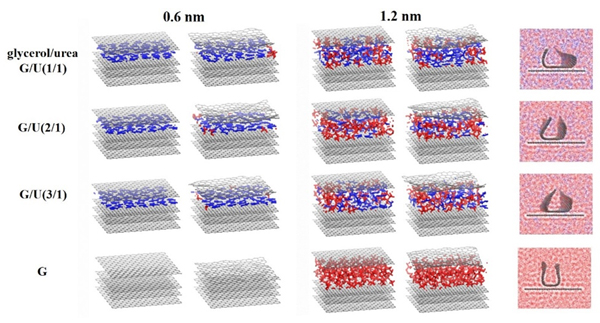
现有的实验方法很难实时观测到石墨烯在液相剥离溶剂中的结构演变, 尤其是石墨烯稳定的微观机理尚不明确. 本工作通过分子动力学方法, 模拟了多层石墨烯和U型石墨烯在不同的物质的量比下的甘油/尿素溶剂中的结构变化, 研究剥离液对石墨烯稳定性的影响. 结果表明, 多层石墨烯在不同溶剂体系中的稳定性差异不显著; 而U型石墨烯在各溶剂体系的稳定性有明显差异, 且稳定能力为: 纯甘油>甘油/尿素(2/1)>甘油/尿素(3/1)>甘油/尿素(1/1). 这说明石墨烯在剥离溶剂中的稳定性与石墨烯的剥离状态有关. 通过溶剂分布发现, 尿素能够进入石墨烯层间, 增加石墨烯层间距; 同时, 甘油能够与尿素形成氢键, 随尿素进入石墨烯层间, 进一步增大层间距, 从而形成稳定的单层或多层受限二元溶剂分子层. 受限溶剂分子层对剥离的石墨烯存在排斥作用, 从而为石墨烯在甘油/尿素二元剥离液中的长期稳定提供了保障.
关键词: 分子动力学, 稳定性, 石墨烯, 甘油, 尿素
Understanding of interfacial structure and stabilization mechanism of graphene sheets in green solvents during liquid-phase exfoliation is of great importance in advancing preparation, characterization and synthesis of graphene-based materials. However, it is difficult to monitor structural evolution of graphene in solvents using current available experimental techniques, and the resulting graphene stabilization mechanism has not been fully understood. In this work, molecular dynamics simulations are performed to investigate the structural evolution and stability behavior of the graphene sheets with different states in the glycerol/urea green solvents with varying concentrations. The results show that only the pristine graphene sheet at an initial interlayer spacing of 0.6 nm in the glycerol solvent restacks back and stays close to each other at a separation close to the intrinsic thickness of graphene. This is due to the fact that the glycerol molecules fail to diffuse into the graphene interlayer, and they could not afford a sufficient repulsive barrier to hinder the graphene aggregation. While in other pristine cases, a single- or double-layer solvent structure is formed and the interlayer separation is maintained at 0.65 nm or 1.12~1.18 nm, offering an efficient dispersion medium to stabilize the graphene sheets. Although the pristine multilayer graphene sheets present similar stability in different glycerol/urea solvents, the U-type graphene experiences distinct levels of stabilization in solvents in the order of pure glycerol>glycerol/urea(2/1)>glycerol/urea(3/1)>glycerol/urea(1/1), signifying that the shifted and exfoliated state of the graphene sheets plays an important role in the stabilization during liquid-phase exfoliation. Moreover, in the glycerol/urea binary solvents, the small urea molecules firstly diffuse into the graphene interlayer due to their strong π-π interaction with graphene, acting as a “dispersion initiator”. And then the glycerol molecules could have the chance to diffuse around or insert into the graphene interlayer to assist in stabilizing the graphene due to the hydrogen bonding between urea and glycerol. In this way, the glycerol helps to further increase the interlayer separation leading to a more stable dispersion, acting as a “dispersion co-stabilizer”. The formation of the confined urea and glycerol solvents thus provides a stable molecular layer structure between the graphene interlayer, enabling to stabilize the exfoliated graphene sheets effectively. As such, the findings in this work are believed to provide the atomic/molecular scale understanding of the stability behavior of the graphene sheets in glycerol/urea binary solvents during liquid-phase exfoliation.
Key words: molecular dynamics, stability, graphene, glycerol, urea
PDF全文下载地址:
点我下载PDF
