摘要/Abstract
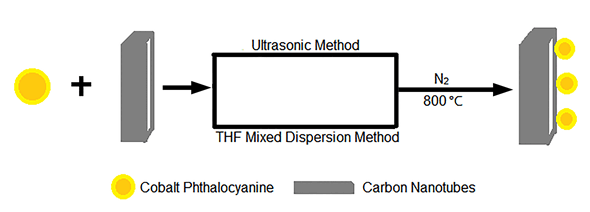
以酞菁钴为催化剂,纳米碳管为载体,分别通过超声法和四氢呋喃法混合分散,并在氮气氛围高温热处理制备了两种酞菁钴催化剂.热重分析(TGA)结果显示超声法制备的酞菁钴催化剂(CoPc-CNT-S)含钴的质量分数为8.1 wt%,四氢呋喃混合法制备的酞菁钴催化剂(CoPc-CNT-R)含钴的质量分数为7.0 wt%.X射线光电子谱(XPS)结果显示CoPc-CNT-R催化剂的含氮量是CoPc-CNT-S催化剂的两倍多,且两种催化剂含氮官能团的种类及比例不同.相比较而言,CoPc-CNT-S表面有更多的吡咯型氮.将两种催化剂应用于混合酸碱燃料电池中发现:CoPc-CNT-S对电催化氧还原有较高的活性和稳定性,将CoPc-CNT-S作为燃料电池的阴极催化剂分别工作5 h和15 h后,电荷转移电阻相对CoPc-CNT-R为阴极催化剂时均较低.原因可能是相近比例的吡啶型N和吡咯型N的协同作用有利于电催化氧还原.
关键词: 非贵金属催化剂, 酞菁钴, 纳米碳管, 燃料电池, 电催化氧还原
An ultrasonic method and a tetrahydrofuran-mixed dispersion method were used to synthesize two heat-treated cobalt phthalocyanine catalysts supported on carbon nanotubes, CoPc-CNT-S and CoPc-CNT-R,respectively. The ultrasonic process was that mixing cobalt phthalocyanine and carbon nanotubes in isopropanol under ultrasound condition within 30 min, while the tetrahydrofuran-mixed dispersion method was that mixing cobalt phthalocyanine and carbon nanotubes in tetrahydrofuran at 80℃ lasting 4 h. Then the pyrolysis process was carried out in a tube furnace under Argon (Ar) atmosphere with a heating rate of 5℃/min to 800℃ and lasting 2 h. Thermogravimetric Analysis (TGA) results showed that cobalt content of CoPc-CNT-S was 8.1 wt% while CoPc-CNT-R was 7.0 wt%. Moreover, X-ray photoelectron spectroscopy (XPS) results gave a conclusion that nitrogen content of CoPc-CNT-R (5.22%) is twice more than CoPc-CNT-S (2.08%). In comparsion with CoPc-CNT-R, CoPc-CNT-S has more pyrrole nitrogen on the surface. The fuel cell tests in a PEM/AEM hybrid fuel cell showed that the activity and stability of CoPc-CNT-S performed better than CoPc-CNT-R. Power density of CoPc-CNT-S hold at 18.6 mW/cm2 in H2/O2 hybrid AEM/PEM fuel cell for 15 h and CoPc-CNT-R can only hold at 9 mW/cm2. The current density of CoPc-CNT-S maintain at 68 mA/cm2 after stability test in H2/O2 hybrid AEM/PEM fuel cell for 20 h under 50 mV, but the stablity of CoPc-CNT-S fluctuate between 20 mA/cm2 to 40 mA/cm2. The reason can be concluded that ultrasonic method and tetrahydrofuran-mixed dispersion method can cause different kind of nitrogen doped on catalyst to influence electrocatalytic properties. The phenomenon that the electron transfer resistance of CoPc-CNT-S was lower than CoPc-CNT-R after working in PEM/AEM fuel cells for 5 h and 15 h can prove indirectly that the activity of CoPc-CNT-R using for the cathode catalyst H2/O2 hybrid AEM/PEM fuel cell is obviously less than CoPc-CNT-S. These observations may result from the cooperative effect from the similar ratio of pyridinic and pyrrolic nitrogen which may accelerate the catalytic activity of CoPc-CNT-S toward oxygen reduction reaction.
Key words: non-noble metal catalysts, cobalt phthalocyanine, carbon nanotubes, fuel cell, oxygen reduction reaction
PDF全文下载地址:
点我下载PDF
