摘要/Abstract
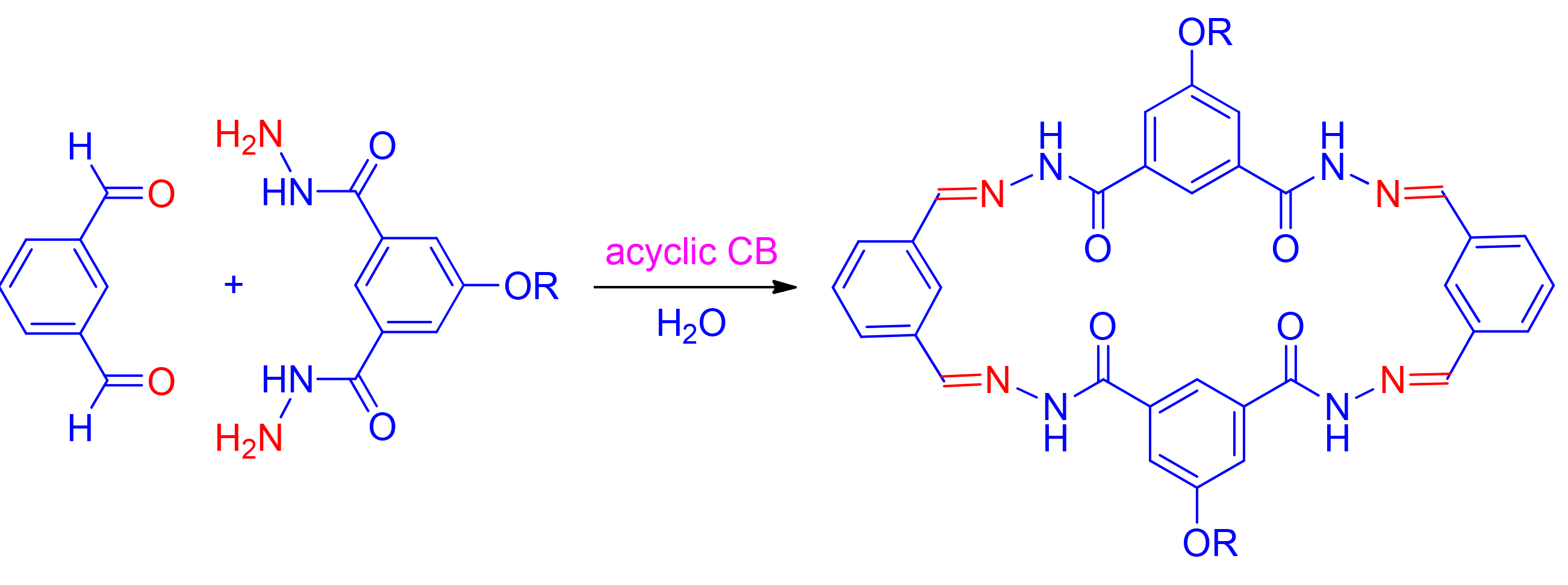
利用核磁实验,研究了并入四个磺酸根或羧酸根的高水溶性开环葫芦脲对疏水性芳烃和芳醛的水相增溶作用.并入四个磺酸根的开环葫芦脲acCB-1能显著提高包括三联苯、4,4'-二甲基联苯和联苯-4,4'-二甲醛在内的芳烃和芳醛的水溶性,其中4,4'-二甲基联苯的溶解度可以提高到8.9 mmol/L,联苯-4,4'-二甲醛的溶解度可以提高到11.2 mmol/L.并入四个羧酸根的acCB-2可以把五氟甲苯和六氟苯在水中的溶解度提高到5.6和3.0 mmol/L.acCB-1还可以通过增溶芳香醛,促进其与酰肼反应形成腙.这一促进作用可以进一步应用于促进两个芳香二醛和一个芳香二酰肼在水中的反应,制备常规条件下不能形成的两个芳香腙大环.
关键词: 开环葫芦脲, 水相增溶, 包结, 芳烃, 芳醛, 腙, 大环化合物
The promotion of two sulfate or carboxylate-bearing acyclic cucurbiturils for the water-solubility of arenes and aromatic aldehydes is described. 1H NMR experiments reveals that sulfate-bearing acyclic cucurbituril (acCB-1) signficantly improves the water-solubility of a number of arenes and aldehydes. For 4,4'-dimethylbiphenyl and biphenyl-4,4'-dicarbaldehyde, the solubility can be improved to 8.9 and 11.2 mmol/L, respectively. 19F NMR experiments demonstrate that carboxylate-bearing acyclic cucurbituril can increase the water-solubilities of pentafluorotoluene and hexafluorobenzene to 5.6 and 3.0 mmol/L, respectively. It is also found that the water-solubilization of acCB-1 for aromatic aldehydes can promote their reaction with acylhydrazines to form hydrozone derivatives. By making use of this promotion, two hydrazone-based macrocycles can be formed from the coupling reactions of two aromatic dialdehdes and one diacylhydrazine in water.
Key words: acyclic cucurbituril, water-solubilization, encapsulation, arene, aromatic aldehyde, hydrazine, macrocycle
PDF全文下载地址:
点我下载PDF
