

1. 重庆医科大学 检验医学院 临床检验诊断学教育部重点实验室,重庆 400016;
2. 重庆理工大学 药学与生物工程学院,重庆 400054
收稿日期:2018-07-31;接收日期:2018-11-26;网络出版时间:2018-01-10
基金项目:国家自然科学基金(Nos. 31570862,81773625)资助
摘要:比较Ni2+-NTA磁珠和羧基磁珠固定结核分枝杆菌二氢叶酸还原酶(Mycobacterium tuberculosis dihydrofolate reductase,MtDHFR),探索适合小分子配体混合物库筛选的MtDHFR固定化方法。重组表达带6×His标签MtDHFR,纯化后表征酶学性质,比较用Ni2+-NTA磁珠和羧基磁珠固定化时相应固定化容量、保留活性、稳定性及对抑制剂响应。结果表明,Ni2+-NTA磁珠对MtDHFR固定化容量为(93±12) mg/g磁珠(n=3),但酶比活保留不超过32%,Ni2+明显抑制酶活性,EDTA与Ni2+呈协同抑制效应,Fe3+无显著干扰。羧基磁珠活化固定MtDHFR的容量(8.6±0.6) mg/g磁珠(n=3),固定化酶比活保留(87±4)% (n=3)。在含50 mmol/L KCl的100 mmol/L HEPES (pH 7.0)中,游离和固定化MtDHFR在0 ℃保存16 h活性都无显著改变,但在25℃保存16 h,游离酶活性下降近60%而羧基磁珠固定化MtDHFR活性下降仅35%。甲氨喋呤对游离MtDHFR和固定化MtDHFR的IC50无显著差异(P > 0.05)。综上,Ni2+-NTA磁珠不适合固定化MtDHFR;羧基磁珠固定化MtDHFR能保留活性、热稳定性及对抑制剂的响应,该固定化方法有望用于快速筛选其配体混合物库。
关键词:磁珠固定化二氢叶酸还原酶配体混合物筛选
Characterization of Mycobacterium tuberculosis dihydrofolate reductase immobilized on magnetic nanoparticles
Wei Zhou1, Jinpeng Lu1, Yaping Li1, Linyu Yang1, Xiaolei Hu1, Fei Liao2, Xiaolan Yang1


1. Key Laboratory of Medical Laboratory Diagnostics of the Ministry of Education of China, College of Laboratory Medicine, Chongqing Medical University, Chongqing 400016, China;
2. School of Pharmacy and Bioengineering, Chongqing University of Technology, Chongqing 400054, China
Received: July 31, 2018; Accepted: November 26, 2018; Published: January 10, 2018
Supported by: National Natural Science Foundation of China (Nos. 31570862, 81773625)
Corresponding author: Xiaolan Yang. Tel: +86-23-68485240; Fax: +86-23-68485239; E-mail: xiaolanyang666@yeah.net.
Abstract: To explore the immobilization of target proteins for screening libraries of ligand mixtures, magnetic submicron particles (MSP) functionalized with Ni2+-NTA and carboxyl were compared for the immobilization of Mycobacterium tuberculosis dihydrofolate reductase (MtDHFR). MtDHFR fused with 6×His was expressed, purified and characterized for kinetics. MtDHFR was immobilized on Ni2+-NTA-functionalized MSP directly and carboxyl-functionalized MSP upon activation. The immobilization capacity, residual activity, thermostability and affinities for putative inhibitors were characterized. MtDHFR immobilized on Ni2+-NTA-functionalized MSP retained about 32% activity of the free one with the immobilization capacity of (93±12) mg/g of MSP (n=3). Ni2+ and EDTA synergistically inhibited MtDHFR activity, while Fe3+ had no obvious interference. MtDHFR immobilized on carboxyl-functionalized MSP retained (87±4)% activity of the free one with the immobilization capacity of (8.6±0.6) mg/g MSP (n=3). In 100 mmol/L HEPES (pH 7.0) containing 50 mmol/L KCl, there was no significant loss of the activities of the free and immobilized MtDHFR after storage at 0 ℃ for 16 h, but nearly 60% and 35% loss of their activities after storage at 25 ℃ for 16 h, respectively. The inhibition effects of methotrexate on the immobilized and free MtDHFR were consistent (P > 0.05). The immobilization of MtDHFR on carboxyl-functionalized MSP was thus favorable for higher retained activity and better thermostability, with promise for rapid screening of its ligand mixtures.
Keywords: magnetic particlesimmobilizationdihydrofolate reductaseligand mixturescreening
结核(Tuberculosis,TB)是由致病性结核分枝杆菌引起的全球流行病[1]。2016年约1 040万人患结核,发病率高于艾滋病,是全球主要的公共卫生问题[2]。现有结核治疗策略面临复发、药物副作用和多药耐药的风险[3-5];应对结核,迫切需要新药[6-7]。二氢叶酸还原酶(Dihydrofolate reductase,DHFR)是细胞核酸代谢途径关键酶,是肿瘤和细菌感染的治疗靶标[8-9]。结核分枝杆菌与人的DHFR氨基酸序列一致性仅为26%左右,基于此结构差异有望设计结核分枝杆菌二氢叶酸还原酶(Mycobacterium tuberculosis DHFR,MtDHFR)选择性抑制剂[10],使MtDHFR成为结核治疗药物的新靶点[11-12]。基于靶蛋白发现配体类药物先导化合物主要依靠筛选配体库。因此,建立适合筛选MtDHFR抑制剂库的技术体系,对发现治疗结核的化学新药具有重要意义。
传统方法筛选配体库需制备纯化合物库再高通量筛选,库制备成本高且效率低,筛选过程耗时且成本高。天然产物混合物和组合合成混合物作为配体库价值很大,而且库制备成本低且制备效率高,但筛选此类混合物库的技术难度巨大[13-15]。药用MtDHFR抑制剂需要具有高亲和力;目前筛选混合库发现高亲和力配体的方法,主要基于亲和结合、靶蛋白配体复合物分离和LC-MS分析[16-18],但也仅限于成分含量相差不大的混合物库。天然产物混合物或组合合成混合物中有效成分含量未知或极低,如何发现极低含量的有效成分仍是技术挑战[19]。本课题组建立了磁分离靶蛋白配体复合物后LC-MS分析筛选混合物组合库的方法[20-21];在此基础上,优化竞争结合反应体系实现选择性迭代富集,可快速发现混合物中极低含量的高亲和力配体;LC-MS为成套的分析系统,靶蛋白大量活性表达、在磁珠表面固定化并保留其活性,就成为应用此策略的关键。
此前发现,对带6×His标签融合蛋白可用Ni2+-NTA磁珠位点选择性固定化以期保留活性[22],但固定化体系必需Ni2+等重金属离子,而这些重金属离子可能影响固定化酶的活性。羧基磁珠也是固定化蛋白的常用载体,此固定化体系基本不会有重金属离子残留干扰酶活性。本研究经重组表达获得MtDHFR,比较Ni2+-NTA磁珠和活化羧基磁珠固定化对MtDHFR活性、稳定性及抑制剂响应的影响,以探索适合磁分离筛选配体混合物库的MtDHFR固定化方案。
1 材料与方法1.1 试剂与器材Ni2+-NTA磁珠(批号20170714,100 g/L)、羧基磁珠(Magnetic submicron particles carboxyl subtype F1,MSP-COOH-F1,批号20171121,120 g/L)购自重庆博蓝鹰生物技术有限公司;三乙醇胺(Trolamine,TEA)、甲氨蝶呤(Methotrexate,MTX)、还原型烟酰胺腺嘌呤二核苷酸磷酸(β-NADPH)、β-巯基乙醇购自Aladdin;六水合氯化镍、硫酸铁、异丙基-β-D-硫代半乳糖吡喃糖苷(Isopropyl-beta-D-thiogalactopyranoside,IPTG)、硫酸卡那霉素、2-吗啉乙磺酸(4-Morpholineethanesulfonic acid,MES)购自北京鼎国昌盛生物技术有限公司;4-羟乙基哌嗪乙磺酸(4-(2-hydroxyerhyl) piperazine-1-erhaesulfonic acid,HEPES)购自北京索莱宝科技有限公司;E. coli BL21 (DE3)购自成都擎科梓熙生物技术有限公司;pET28a购自中美泰和生物技术有限公司;Ni2+-NTA层析柱购自南京金斯瑞生物科技有限公司;二氢叶酸(Dihydrofolic acid,DHF)购自Sigma公司;N-羟基琥珀酸亚胺(N-Hydroxysuccinimide,NHS)、1-(3-二甲基氨基丙基)-3-乙基碳二亚胺(1-(3-Dimethylaminopropyl) -3-ethylcarbodiimide,EDC)购自东京化成工业株式会社,其他试剂均为国产分析纯。日本岛津UV-2550紫外可见分光光度计(带恒温系统);其林贝尔QB-9001快速振荡器;Promega 12孔磁分离架;Millipore 8050型超滤杯及再生纤维素超滤膜(截留蛋白分子量 > 10 kDa)。
1.2 MtDHFR重组表达纯化及表征1.2.1 MtDHFR重组表达纯化含N端携带6×His标签的pET28a(+):dfrA表达质粒[23]委托中美泰和生物技术有限公司合成。质粒转化至感受态E. coli BL21 (DE3)培养,经单克隆测序鉴定后,于含有100 mg/L卡那霉素LB液体培养基中,参照文献[23]进行6×His-MtDHFR表达和纯化。用含50 mmol/L KCl的20 mmol/L磷酸钾缓冲液(pH 7.0)氮气正压(0.15-0.20 Mpa)超滤浓缩收集蛋白,Bradford法测蛋白浓度[24],15% SDS-PAGE分析蛋白纯度;所得酶蛋白溶液于?80 ℃保存。
1.2.2 MtDHFR的酶学性质表征MtDHFR活性测定:DHF用含10 mmol/L β-巯基乙醇的50 mmol/L磷酸钾缓冲液(pH 7.0)溶解至1.0 mmol/L溶液,β-NADPH用去离子水溶解至1.0 mmol/L溶液;反应缓冲液为含50 mmol/L KCl的100 mmol/L HEPES (pH 7.0) [25]。MtDHFR用反应缓冲液稀释至40 mg/L。紫外分光光度计恒温至25 ℃,反应缓冲液调零,反应总体积为1.0 mL,加终浓度均为50 μmol/L的β-NADPH和DHF,400 ng MtDHFR,混匀后延迟10 s用光度计连续监测340 nm处3 min内的消光变化;取消光变化线性范围内的速度(ΔA/min)作为初始反应速度(V),按设定的底物消耗消光系数(ε= 11 800 L/(mol·cm)[26]计算酶活力,1.0 min内转化1.0 μmol底物所需酶量为一个活力单位。
MtDHFR米氏常数:文献报道β-NADPH对MtDHFR的米氏常数KmNADPH和DHF的米氏常数KmDHF均约4 μmol/L[27]。对于双底物酶,使其中一个底物浓度大于10 Km时改变另一底物浓度测定酶活,双倒数分析确定表观Km[28]。在1.0 mL含400 ng酶测定体系中,固定DHF终浓度为50 μmol/L,在2-10 μmol/L间改变β-NADPH终浓度(S),测定初始反应速度(V),回归分析1/V对β-NADPH浓度倒数1/S响应得KmNADPH;固定β-NADPH终浓度50 μmol/L,在(0.5-10) μmol/L间改变DHF浓度,同法测定KmDHF。
MtDHFR热稳定性:室温筛选配体操作过程不超过4 h[20-21]。用酶反应缓冲液将MtDHFR稀释到0.1 g/L,于25 ℃和0 ℃保存,不同时刻取MtDHFR溶液10 μL,25 ℃测定酶活分析其变化。
1.3 Ni2+-NTA磁珠固定MtDHFR及表征1.3.1 Ni2+-NTA磁珠固定MtDHFR取Ni2+-NTA磁珠悬液(100 g/L),磁力回收磁珠后用固定缓冲液(pH 7.0,20 mmol/L Tris-HCl)洗3次并重悬为7.5 g/L。取不同浓度MtDHFR溶液40 μL预冷到0 ℃,200 r/min缓慢加入10 μL Ni2+-NTA磁珠(约75 μg),冰水浴中200 r/min振摇30 min;磁力分离取上清备测剩余蛋白,磁珠用固定缓冲液小心洗涤3次,每次200 μL,再重悬为3 g/L,于0 ℃保存待用。
1.3.2 Ni2+-NTA磁珠固定MtDHFR的容量及固定酶活性用Bradford法测酶蛋白量[24]。固定时所用酶蛋白总量与上清酶蛋白量之差为固定化酶量。取饱和固载时单位量Ni2+-NTA磁珠固定酶量为固定化容量。用终点法测定固定化MtDHFR活性;在25 ℃恒温的1.0 mL酶反应体系中,加30 μg固定化MtDHFR的磁珠,反应3 min后分离全部磁珠测定上清消光,以不加固定酶磁珠为对照计算消光之差及其变化速度(ΔA/min)。
1.3.3 Ni2+、Fe3+及EDTA对MtDHFR活性的影响将NiCl2、Fe2(SO4)3、EDTA用酶反应缓冲液配成5 mmol/L溶液。在1.0 mL酶反应缓冲液中,加不同终浓度的Ni2+、EDTA、Fe3+,分别与400 ng MtDHFR在冰上200 r/min作用不同时间;Ni2+ 与EDTA作用20 min后再加MtDHFR 400 ng冰上作用不同时间;然后恒温25 ℃加底物测定酶活性。
1.4 羧基磁珠固定MtDHFR及表征1.4.1 活化羧基固定MtDHFR取MSP-COOH-F1悬液(120 g/L),用10 mmol/L MES缓冲液(pH 6.0)的洗3次并重悬至3 g/L。NHS和EDC分别用预冷10 mmol/L MES (pH 6.0)配成75 mmol/L、50 mmol/L溶液。取200μL磁珠悬液(约600 μg),磁力分离去上清后加NHS和EDC各100 μL (摩尔比1.5:1.0)[29],或各50 μL再补充缓冲液至总体积200 μL,室温200 r/min振摇反应30 min后磁力回收磁珠,用预冷到0 ℃的固定缓冲液(pH 7.0的10 mmol/L MES)洗3次并重悬至15 g/L,于0 ℃保存(尽快使用)。在预冷到0 ℃含一定量MtDHFR的210 μL固定缓冲液中,分批加入预冷的活化羧基磁珠40μL (600 μg);在0 ℃固定反应30 min,每隔3 min混匀一次。磁力回收磁珠,用酶反应缓冲液洗涤并重悬至3 g/L,于0 ℃保存备用。参照Ni2+-NTA磁珠固定化酶方法测定固定酶量。
1.4.2 光度法连续跟踪磁珠固定酶反应过程测定MtDHFR活性于1.0 mL酶反应体系中,分别在含底物50 μmol/L DHF、50 μmol/L β-NADPH的反应缓冲液中加240 μg MSP-COOH-F1及1.0 μg酶,25 ℃下比较底物加磁珠前后340 nm消光值A340及其在酶作用下变化速度(ΔA/min),以消除磁珠对测定消光变化速度的影响。测定磁珠固定化酶活性时,在25 ℃的1.0 mL酶反应体系中加75 μg固定酶磁珠,以10 s间隔连续监测3 min内340 nm处消光变化,并计算消光变化速度(ΔA/min)及对应酶活性。
1.4.3 羧基磁珠固定化MtDHFR和游离MtDHFR对MTX的响应于1.0 mL酶反应体系中,加75 μg固定酶磁珠或0.5 μg游离酶,及不同终浓度MTX (1.0-10.0 nmol/L),25 ℃混匀3 min后加底物测定酶活。用OriginPro 9.1拟合抑制率对MTX浓度对数响应确定甲氨蝶呤对MtDHFR的IC50。
1.4.4 羧基磁珠固定MtDHFR储存稳定性将固定酶磁珠(3.0 g/L)于25 ℃水浴和冰水浴0 ℃中静置保存,于不同时刻取75 μg磁珠测定酶活性;分析活性随保存时间的变化。
1.5 数据处理方法每个实验重复3次,实验结果表示为平均值±标准偏差(x±s);采用OriginPro 9.1进行数据处理;统计学处理采用SPSS 20.0,t检验分析,P < 0.05为具有显著性差异。
2 结果与分析2.1 MtDHFR表达和纯化6×His-MtDHFR诱导表达后,粗酶液经Ni2+-NTA亲和层析单次纯化,蛋白收率约为12%,比活提高到15倍;超滤浓缩3次,蛋白收率约为30%,但比活提高近30%。纯化总倍数约21倍(表 1)。可见,高纯度MtDHFR易于获得。SDS-PAGE显示较纯的目的蛋白条带(图 1),分子量约为20 kDa,符合预期。
表 1 MtDHFR表达纯化效果(x±s, n=3)Table 1 Expression and purification of MtDHFR (x±s, n=3)
| Purification steps | Total protein (mg) | Total activity (U) | Specific activity (U/mg) | Purified fold | Recovery rate (%) |
| Crude extract | 135±12 | 30±1.5 | 0.2±0.1 | ||
| Purification from Ni2+-NTA | 16±1.2 | 50±4.0 | 3.1±0.2 | 15.5 | 12 |
| Ultra-filtration | 4.8±0.4 | 20±1.7 | 4.2±0.2 | 21.0 | 30 |
表选项
 |
| 图 1 SDS-PAGE检测6×His-MtDHFR的纯化 Fig. 1 SDS-PAGE analysis of 6×His-MtDHFR after purification by Ni2+-NTA. Lane 1: lysate; lane 2: supernatant; lane 3: sediment; lane 4: flow through fractions; lane 5: fractions eluted with 10 mmol/L imidazole; lane 6: fractions eluted with 20 mmol/L imidazole; lane 7: fractions of target eluted with 500 mmol/L imidazole; M: marker. |
| 图选项 |
2.2 MtDHFR米氏常数及其受pH的影响2.2.1 MtDHFR米氏常数双倒数分析得Km DHF为(4.4±0.2) μmol/L (n=3) (图 2A),Km NADPH为(4.7±0.5)μmol/L (n=3) (图 2B),都与文献报道[27]接近。
 |
| 图 2 DHF (A)和β-NADPH (B)的表观Km Fig. 2 Km of DHF (A) and β-NADPH (B). |
| 图选项 |
2.2.2 pH对MtDHFR活性影响在pH 4.0-8.0的50 mmol/L磷酸盐缓冲液中,MtDHFR活性随pH增大而降低,到pH 8.0时几乎无活性(图 3)。MtDHFR活性在pH 7.0的100 mmol/L HEPES和pH 7.0的50 mmol/L磷酸盐中无差异,故用pH 7.0的HEPES测定酶活性[25]。
 |
| 图 3 MtDHFR活性的pH效应 Fig. 3 pH effect on MtDHFR activity. |
| 图选项 |
2.3 Ni2+-NTA磁珠固定MtDHFR及其表征2.3.1 Ni2+-NTA磁珠固定MtDHFR容量及保留活性用75 μg Ni2+-NTA磁珠,随MtDHFR用量增加,固定化酶量逐渐增加直至饱和(图 4),固定化容量为(93±12) mg/g磁珠(n=3)。固定化酶比活随MtDHFR用量增加而缓慢增加至稳定,但最大比活保留仅约为游离酶的32%。
 |
| 图 4 MtDHFR活性的pH效应75 μg Ni2+-NTA磁珠基于6×His固定MtDHFR的固定化量和表观比活保留比 Fig. 4 Immobilization capacity and residual activity percentage of 6×His-MtDHFR on Ni2+-NTA MSPs of 75 μg. |
| 图选项 |
2.3.2 Ni2+、Fe3+及EDTA对酶活的影响Ni2+在5-20 nmol/L之间对MtDHFR活性产生浓度和时间依赖性抑制,20 nmol/L Ni2+抑制大于50% (图 5A)。Fe3+对MtDHFR活性没有明显影响(图 5B)。单独EDTA对MtDHFR活性可抑制大于30%且依赖其浓度;Ni2+与MtDHFR作用前后加EDTA对MtDHFR活性抑制大于60%,有协同抑制效应(图 5C)。Ni2+-NTA磁珠中含游离Ni2+,其可能降低固定酶活力且EDTA不能逆转。
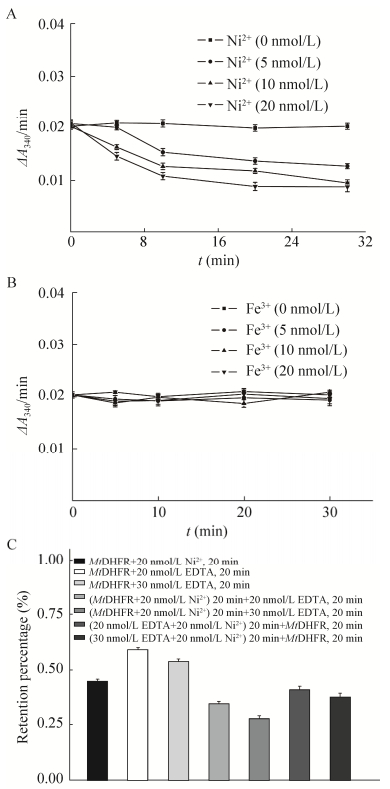 |
| 图 5 Ni2+(A)、Fe3+(B)、EDTA (C)对酶活性的影响 Fig. 5 Effects of Ni2+, Fe3+and EDTA on MtDHFR activity. (A) Ni2+. (B) Fe3+. (C) EDTA alone and it plus Ni2+ in 100 mmol/L HEPES buffer at pH 7.0. |
| 图选项 |
2.4 羧基磁珠固定MtDHFR及其表征2.4.1 羧基磁珠对酶活测定的干扰常规方法测定磁珠固定化酶活,需在固定化酶初速度反应阶段终止反应并分离磁珠,再测定反应液的吸收变化计算固定化酶活。由于反应终点的确定易产生误差并干扰反应液吸收的测定,使固定化酶活测定重现性较低,故需考察不分离磁珠连续监测反应测定酶活的可行性。所用磁珠悬浮稳定性好,其在有限时段内对340 nm消光变化速度影响应该很小。在1.0 mL酶反应体系中,150 mg/L羧基磁珠、50 μmol/L β-NADPH和50 μmol/L DHF及其混合物,在340 nm的消光A340构成恒定本底;在游离酶存在时,外加终浓度75、150、240 mg/L羧基磁珠,对光度法跟踪酶反应过程测定游离酶活性无影响。因此,磁珠对固定酶活测定无显著干扰。本实验限制固定酶磁珠用量,用光度法连续跟踪测定固定化酶活性。
2.4.2 羧基磁珠固定MtDHFR容量及保留活性活化羧基磁珠约600 μg,MtDHFR用量在5-200 μg之间,固定化容量基本不变(图 6);最大固定化蛋白量约5.2 μg,对应固定化容量为(8.6±0.6) mg/g磁珠(n=3)。不分离磁珠,连续跟踪固定化酶反应过程并测定固定化酶活性,所得固定化酶表观比活保留比例变化如图 6所示。在约600 μg活化羧基磁珠中,加入MtDHFR量从5-200 μg进行固定时,磁珠固定化酶的表观比活保留比例先增加后逐渐降低至稳定,在酶用量10 μg时其表观比活保留87%,而酶用量20-200 μg时磁珠固定化酶表观比活保留比例稳定在75%。为保留固定酶活性,选择酶量:磁珠量比为1:60。
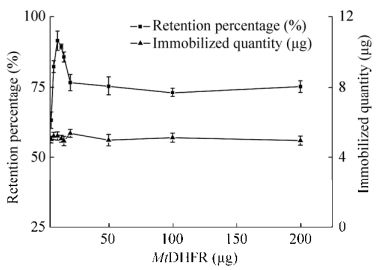 |
| 图 6 羧基磁珠固定酶活保留比例及固定化量对加入酶量的响应 Fig. 6 Immobilized quantity and retention percentages of apparent specific activities in response to amounts of enzyme added for immobilization on MSP-COOH-F1. |
| 图选项 |
2.4.3 游离和羧基磁珠固定MtDHFR的储存稳定性游离和羧基磁珠固定化MtDHFR在反应缓冲液0 ℃保存16 h活性均无显著改变(图 7);游离MtDHFR在25 ℃下反应缓冲液中保存2 h后活性持续降低,4 h下降超过10%,16 h降低近60%;固定化酶25 ℃保存4 h活力仅下降4%,16 h下降仅35%。在混合物库筛选中,从配体竞争结合固定化酶到磁分离洗涤的全流程,操作时间不超过4 h,故此固定化MtDHFR稳定性满足混合物筛选要求。
 |
| 图 7 游离酶和羧基磁珠固定MtDHFR的稳定性 Fig. 7 Stability of the free and MSP-COOH-F1 immobilized MtDHFR. |
| 图选项 |
2.4.4 MtDHFR羧基磁珠固定前后对MTX的IC50MtDHFR在羧基磁珠上固定前后对MTX的IC50无显著差异(P > 0.05) (图 8),且都与文献报道结果接近[25, 27]。可见,用MSP-COOH-F1固定化MtDHFR适合筛选其高亲和力抑制剂。
 |
| 图 8 MTX对游离酶和羧基磁珠固定MtDHFR的IC50 Fig. 8 IC50 of MTX against free and MSP-COOH-F1 immobilized MtDHFR. |
| 图选项 |
3 讨论本研究构建N端带6×His标签的MtDHFR表达载体pET28a,在E. coli BL21 (DE3)中成功重组表达并纯化。比较发现,不同磁珠固定化重组MtDHFR有显著差异。6×His-MtDHFR通过其6×His标签与Ni2+-NTA磁珠螯合,属于位点选择性固定化,但不适合用于磁分离筛选MtDHFR的高亲和力配体。首先,Ni2+-NTA磁珠固定化酶保留活性很低。其次,Ni2+-NTA鳌合带6×His标签酶复合物稳定性对pH敏感,在pH 8.0及以上鳌合酶才能维持固定化状态。但是,6×His-MtDHFR在pH 8.0时保留的活性很低而不适合筛选其抑制剂。相反,MtDHFR氨基酸序列仅有1个赖氨酸(Lys-53)及N端伯氨基,三维结构(PDB code: 4KNE)中,此赖氨酸及其N端伯氨基都远离活性位点[23],可通过伯氨基与磁珠表面羧基生成酰胺固定化;这种固定化实际上也属于位点选择性固定化。MSP-COOH-F1表面为兼性离子对修饰层并带长连接臂的羧基适合固定蛋白,而磁珠对疏水小分子的非特异吸附弱。优化条件后,此羧基磁珠固定MtDHFR达到(8.6±0.6) mg/g磁珠,特别是固定化酶保留(87±4)%活性,固定前后其对MTX的IC50无显著差异,而固定化酶在4 h内稳定。所以,用此羧基磁珠固定化MtDHFR有望用于配体混合物库中高亲和力配体的选择性富集与筛选。
用MSP-COOH-F1羧基磁珠固定化MtDHFR时,磁珠表面羧基活化程度不宜太高,对设定量磁珠存在最优酶蛋白用量。可能磁珠固定蛋白量少而使位阻小,有利于固定化酶结合底物。对MSP-COOH-F1,酶蛋白量对磁珠量比例接近1:60,有利于获得高保留活性固定化酶。MSP-COOH-F1羧基固定化MtDHFR后悬浮稳定性好,光度法连续监测反应混合物消光(考虑磁珠对光的散射故称为消光)适合测定酶活性。
总体而言,6×His-MtDHFR适合在大肠杆菌大量表达,通过成酰胺键固定在MSP-COOH-F1上适用于磁分离筛选其抑制剂混合物库。后续工作正在进行中。
参考文献
| [1] | Zumla A, George A, Sharma V, et al. WHO's 2013 global report on tuberculosis: successes, threats, and opportunities.Lancet, 2013, 382(9907): 1765–1767.DOI: 10.1016/S0140-6736(13)62078-4 |
| [2] | The World Health Organization (WHO). Global tuberculosis report 2018[EB/OL]. 2018-09-18. http://www.who.int/tb/publications/global_report/en/. |
| [3] | Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis.Lancet, 2010, 375(9728): 1830–1843.DOI: 10.1016/S0140-6736(10)60410-2 |
| [4] | Koul A, Arnoult E, Lounis N, et al. The challenge of new drug discovery for tuberculosis.Nature, 2011, 469(7331): 483–490.DOI: 10.1038/nature09657 |
| [5] | Chetty S, Ramesh M, Singh-Pillay A, et al. Recent advancements in the development of anti-tuberculosis drugs.Bioorg Med Chem Lett, 2017, 27(3): 370–386.DOI: 10.1016/j.bmcl.2016.11.084 |
| [6] | Palomino JC, Martin A. Is repositioning of drugs a viable alternative in the treatment of tuberculosis?.J Antimicrob Chemother, 2013, 68(2): 272–283. |
| [7] | Nguyen L, Jacobs MR. Counterattacking drug-resistant tuberculosis: molecular strategies and future directions.Expert Rev Anti Infect Ther, 2012, 10(9): 959–961.DOI: 10.1586/eri.12.97 |
| [8] | Sharma M, Chauhan PM. Dihydrofolate reductase as a therapeutic target for infectious diseases: opportunities and challenges.Future Med Chem, 2012, 4(10): 1335–1365.DOI: 10.4155/fmc.12.68 |
| [9] | Rashid N, Thapliyal C, Chaudhuri P. Dihydrofolate reductase as a versatile drug target in healthcare.J Proteins Proteomics, 2016, 7(4): 247–257. |
| [10] | El-Hamamsy MHRI, Smith AW, Thompson AS, et al. Structure-based design, synthesis and preliminary evaluation of selective inhibitors of dihydrofolate reductase from Mycobacterium tuberculosis.Bioorg Med Chem, 2007, 15(13): 4552–4576.DOI: 10.1016/j.bmc.2007.04.011 |
| [11] | Yang X, Wedajo W, Yamada Y, et al. 1, 3, 5-triazaspiro[5.5]undeca-2, 4-dienes as selective Mycobacterium tuberculosis dihydrofolate reductase inhibitors with potent whole cell activity.Eur J Med Chem, 2018, 144: 262–276.DOI: 10.1016/j.ejmech.2017.12.017 |
| [12] | Santa Maria JP Jr, Park Y, Yang LH, et al. Linking high-throughput screens to identify MoAs and novel inhibitors of Mycobacterium tuberculosis dihydrofolate reductase.ACS Chem Biol, 2017, 12(9): 2448–2456.DOI: 10.1021/acschembio.7b00468 |
| [13] | Bindseil KU, Jakupovic J, Wolf D, et al. Pure compound libraries; a new perspective for natural product based drug discovery.Drug Discovery Today, 2001, 6(16): 840–847.DOI: 10.1016/S1359-6446(01)01856-6 |
| [14] | Su YH, Chiang LW, Jeng KC, et al. Solution-phase parallel synthesis and screening of anti-tumor activities from fenbufen and ethacrynic acid libraries.Bioorg Med Chem Lett, 2011, 21(5): 1320–1324.DOI: 10.1016/j.bmcl.2011.01.068 |
| [15] | Mishra BB, Tiwari VK. Natural products: an evolving role in future drug discovery.Eur J Med Chem, 2011, 46(10): 4769–4807.DOI: 10.1016/j.ejmech.2011.07.057 |
| [16] | Annis DA, Nickbarg E, Yang XS, et al. Affinity selection-mass spectrometry screening techniques for small molecule drug discovery.Curr Opin Chem Biol, 2007, 11(5): 518–526.DOI: 10.1016/j.cbpa.2007.07.011 |
| [17] | Macarron R, Banks MN, Bojanic D, et al. Impact of high-throughput screening in biomedical research.Nat Rev Drug Discov, 2011, 10(3): 188–195.DOI: 10.1038/nrd3368 |
| [18] | Holdgate GA, Anderson M, Edfeldt F, et al. Affinity-based, biophysical methods to detect and analyze ligand binding to recombinant proteins: matching high information content with high throughput.J Struct Biol, 2010, 172(1): 142–157.DOI: 10.1016/j.jsb.2010.06.024 |
| [19] | Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review.Biotechnol Adv, 2015, 33(8): 1582–1614.DOI: 10.1016/j.biotechadv.2015.08.001 |
| [20] | Yang XL, Xie YL, Pu J, et al. Estimation of affinities of ligands in mixtures via magnetic recovery of target-ligand complexes and chromatograpHic analyses: chemometrics and an experimental model.BMC Biotechnol, 2011, 11(1): 44.DOI: 10.1186/1472-6750-11-44 |
| [21] | Yang XL, Pu J, Zhao H, et al. Method to screen aromatic ligands in mixtures for quantitative affinities to target using magnetic separation of bound ligands along with HPLC and UV photometry detection.Microchim Acta, 2012, 176(1/2): 243–249. |
| [22] | Yang XL, Hu XL, Chen CY, et al. Comparison of the immobilization of 6His-tagged proteins on magnetic-submicron-particle functionalized with Ni2+-NTA and bis-sulfone.Nanosci Nanotech Lett, 2015, 7(6): 486–494.DOI: 10.1166/nnl.2015.1996 |
| [23] | Dias MVB, Tyrakis P, Domingues RR, et al. Mycobacterium tuberculosis dihydrofolate reductase reveals two conformational states and a possible low affinity mechanism to antifolate drugs.Structure, 2014, 22(1): 94–103.DOI: 10.1016/j.str.2013.09.022 |
| [24] | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal Biochem, 1976, 72(1/2): 248–254. |
| [25] | Nixon MR, Saionz KW, Koo MS, et al. Folate pathway disruption leads to critical disruption of methionine derivatives in Mycobacterium tuberculosis.Chem Biol, 2014, 21(7): 819–830.DOI: 10.1016/j.chembiol.2014.04.009 |
| [26] | Stone SR, Morrison JF. Kinetic mechanism of the reaction catalyzed by dihydrofolate reductase from Escherichia coli.Biochemistry, 1982, 21(16): 3757–3765.DOI: 10.1021/bi00259a006 |
| [27] | White EL, Ross LJ, Cunningham A, et al. Cloning, expression, and characterization of Mycobacterium tuberculosis dihydrofolate reductase.FEMS Microbiol Lett, 2004, 232(1): 101–105.DOI: 10.1016/S0378-1097(04)00038-2 |
| [28] | Cleland WW. The statistical analysis of enzyme kinetic data.Adv Enzymol Relat Areas Mol Biol, 1967, 29: 1–32. |
| [29] | Puertas S, Batalla P, Moros M, et al. Taking advantage of unspecific interactions to produce highly active magnetic nanoparticle-antibody conjugates.ACS Nano, 2011, 5(6): 4521–4528.DOI: 10.1021/nn200019s |
