

兰州大学 细胞活动与逆境适应教育部重点实验室,甘肃 兰州 730000
收稿日期:2019-04-25;接收日期:2019-07-23;网络出版时间:2019-08-06
基金项目:兰州大学中央高校基本科研业务费专项资金(No. lzujbky-2018-105)资助
作者简介:刘璞 ??兰州大学生命科学学院副教授。近年一直从事利用微生物资源来减除环境污染物如重金属、抗生素等对生物的危害的机理与应用研究。已在Journal of Hazardous Materials、Nutrients等期刊上发表相关SCI论文20余篇,申请国内发明专利3项。本课题组所属的环境微生物研究团队结合近年研究成果,提出了应对环境污染物的“肠道修复”策略,获得了领域内专家的认可.
摘要:近年来随着抗生素在畜牧业、水产养殖业以及医疗行业的广泛应用,大量抗生素通过排泄物进入环境,导致我国大面积水体及土壤环境中抗生素残留量急剧增高。环境中不同种类的抗生素的残留导致微生物种群结构失衡,对生态环境及人类造成极大危害。因此,解决抗生素残留问题是21世纪新型环境污染物领域的一个重要课题。已有研究显示,一些微生物能够以抗生素为碳源生存,可用于降解环境中残留抗生素,但人们对微生物降解抗生素的降解机制了解较少。文中概括了近十年来抗生素降解菌株和菌群对抗生素的去除情况,以及应用微生物菌群处理抗生素残留的技术方法,同时对未来利用微生物修复法减少环境中抗生素残留进行了展望。
关键词:抗生素生物降解菌株微生物群落合成生物学
Progress in microbial remediation of antibiotic-residue contaminated environment
Ying Wu, Pengya Feng, Rong Li, Xiao Chen, Xiangkai Li, TAWATCHAI SUMPRADIT, Pu Liu


Key Laboratory of Cell Activity and Stress Adaptation, Lanzhou University, Lanzhou 730000, Gansu, China
Received: April 25, 2019; Accepted: July 23, 2019; Published: August 6, 2019
Supported by: Fundamental Research Funds for the Central Universities (No. lzujbky-2018-105)
Corresponding author: Pu Liu, Tel: +86-931-8912560; E-mail: liupu@lzu.edu.cn.
Abstract: In recent years, antibiotics have been widely used in animal husbandry, aquaculture and the medication in China. Many antibiotics are discharged into the environment, resulting in dramatic increase of antibiotic residues in domestic water and soil. Residues of different antibiotics in the environment change the microbial structure, which is extremely harmful to the ecological environment and humans. Therefore, remediation of antibiotic contamination is significantly important. Studies have shown that some microorganisms can degrade and utilize antibiotics, and thus have good application prospects on bioremediation of antibiotic contamination. However, little is known about the microbial degradation mechanism of antibiotics. This article summarizes the removal of antibiotics by antibiotic-degrading strains and bacterial flora in recent ten years, and the methods of using microbial flora to treat antibiotic residues. The future prospect of using microbial remediation to reduce antibiotic residues in the environment has also been discussed.
Keywords: antibioticsbiodegradationstrainsmicrobial communitiessynthetic biology
自1928年弗莱明发现青霉素后,抗生素作为一种新的应对病原体感染的方式被广泛应用于医学、畜牧、水产养殖等领域[1-2]。抗生素是具有抗菌活性的天然、合成和半合成化合物,能对其他活性细胞的发育产生干扰作用,主要包括磺胺类、四环素类、β-内酰胺类、氟喹诺酮类、大环内脂类等。中国是世界上使用抗生素最频繁的国家之一,调查显示,2013年我国抗生素的使用量达到16万t,其中52%用于畜牧业;2018年,我国约70%的住院病人和20%的门诊病人使用抗生素类药物,约为发达国家使用率的两倍。抗生素在人类疾病治疗和畜禽生产等方面作出巨大贡献的同时也带来了新的问题。有数据表明,2001年至2005年间,约有60万名患者死于抗生素滥用[3]。研究还发现,动物体摄入的磺胺类药物中约有50%不经修饰直接排放至水体和土壤环境中,进而对人类和生态系统产生潜在的威胁[4],例如导致多种耐药致病菌的出现、抗生素抗性基因的扩散等[5-6]。因此,抗生素广泛使用带来的环境污染问题已成为新型有机污染物领域的重要课题。
近年来,研究者对去除环境中抗生素残留的问题已进行了大量的研究,目前已将理化方法、微生物降解法应用于环境中抗生素的去除。理化方法如活性炭吸附法、低温等离子技术、土壤渗滤系统法和超声降解法等,主要用于去除环境中的有机污染物,在抗生素去除方面的应用存在成本较高、去除效率低的问题[7]。微生物降解法作为一种去除环境抗生素残留的生物方法,具有成本低、效能高以及环境污染小等特点,是处理抗生素污染的有效途径之一[8]。本文对近年来抗生素降解菌株和菌群及其在去除环境中抗生素残留方面的应用进行了系统综述,并对部分抗生素的微生物降解机制进行总结,为今后抗生素的微生物修复研究提供参考。
1 降解抗生素的菌株、降解途径和应用现状1.1 降解抗生素的菌株1.1.1 降解抗生素的细菌抗生素的生物降解以微生物代谢为主。近年来通过筛选、富集和驯化等方式分离获得了许多具有抗生素降解能力的细菌菌株(表 1),这些细菌参与分解的抗生素包括磺胺类(Sulfonamide antibiotics, SAs)、四环素类(Tetracycline antibiotics, TCs)、氟喹诺酮类(Fluoroquinolones, FQ)、大环内脂类(Macrolides antibiotics, MA)、β-内酰胺类和多肽等五类,其中以SAs和TCs为主,可能与其在环境中的残留量有关[15, 31]。获得的具有抗生素降解能力的细菌来自无色杆菌属、产碱杆菌属、微杆菌属、氨氧化细菌、苍白杆菌属、戈登式菌属、葡萄球菌属、鞘氨醇菌属、栖热菌属、拉乌尔菌属、伯克氏菌属、芽孢杆菌属和假单胞菌属等。这些细菌是土壤、污水中的常见菌株,80%属于厚壁菌门和变形菌门,少部分来自栖热菌门、拟杆菌门、放线菌门和浮霉菌门等。
表 1 近五年文献中的抗生素特异性降解细菌Table 1 Antibiotic-degrading bacterial strains reported in recent 5 years
| Antibiotics | Bacterial species | Concentration (mg/L) | Removal rate (%) | Sources | References | |
| Sulfonamides | Sulfamethoxazole | Microbacterium sp. BR1 | 100 | 40–60 (1 h) | Laboratory | [9] |
| Sulfamethoxazole | Nitrosomonas Ammonia oxidizing bacteria | 100 | 86 (70 d) | Domestic wastewater | [10] | |
| Sulfamethoxazole | Achromobacter sp. S-3 | 100 | 80 (25 d) | Aerobic sludge | [11] | |
| Sulfamethoxazole | Achromobacter denitrificans PR1 | 250 | 44 (16 h) | Activated sludge wastewater | [4] | |
| Sulfamethoxazole | Pseudomonas mandelii McBPA4 | 50 | 73 (15 d) | Laboratory | [12] | |
| Sulfamethoxazole | Acinetobacter sp. W1 | 5–240 | 100 (10 h) | Activated sludge | [13] | |
| Sulfadimidine | Bacillus cereus J2 | 50 | 100 (36 h) | Municipal sewage | [14] | |
| Sulfamethoxazole | Ochrobactrum sp. SMX-PM1-SA1 | 5 | 45.2 (8 d) | Wastewater | [15] | |
| Sulfamethoxazole | Labrys sp. SMX-W11 | 5 | 62.2 (8 d) | Activated sludge | ||
| Sulfamethoxazole | Gordonia sp. SMX-W2-SCD14 | 5 | 51.4 (8 d) | Pig manure | ||
| Tetracyclines | Tetracycline | Advenella sp.40 < 02 | 50 | 57.8 (5 d) | Factory | [16] |
| Tetracycline | Stenotrophomonas maltophilia DT1 | 10 | 70 (3 d) | Factory sewage | [17] | |
| Tetracycline | Raoultella sp. XY-1 | 20 | 70.68 (8 d) | Pig farm sediment | [18] | |
| Tetracycline | Sphingobium sp. PHE3 | 20 | 40.1 (90 d) | Contaminated farmland soil | [19] | |
| Oxytetracycline | Pseudomonas sp. T4 | 100 | 26.88 (7 d) | Livestock manure | [20] | |
| Oxytetracycline | Ochrobactrum sp. KSS10 | 30 | 63.33 (4 d) | Municipal sludge | [21] | |
| Oxytetracycline | Achromobacter sp. TJ-2# | 50 | 58.3 (3 d) | Contaminated soil | [22] | |
| Tetracycline | Achromobacter sp. TJ-2# | 50 | 63.9 (3 d) | |||
| Aureomycin | Achromobacter sp. TJ-2# | 50 | 65.5 (3 d) | |||
| Fluoroquinolones | Ciprofloxacin | Thermus thermophiles C419 | 5 | 57 (5 d) | Pharmaceutical sludge | [23] |
| Norfloxacin | Staphylococcus caprae NOR-36 | 5 | 92.6 (10 d) | Pharmaceutical wastewater | [24] | |
| Ofloxain | Labrys portucalensis F11 | 0.45 | 34.6 (28 d) | Fluorobenzene | [25] | |
| Ofloxain | Rhodococcus sp. FR1 | 0.45 | 39.3 (28 d) | |||
| Beta-lactam | Cefalexin | Pseudomonas sp. CE21 | 10 | 46.7 (1 d) | Activated sludge | [26] |
| Cefalexin | Pseudomonas sp. CE22 | 10 | 90 (1 d) | Activated sludge | [26] | |
| Cefalexin | Shewanella strain | 5 | 47.9 (40) | EBPR SBR system | [27] | |
| Amoxicillin | Shewanella strain | 10 | 90 (40) | EBPR SBR system | [27] | |
| Macrolides | Tylosin | Achromobacter | 50 | 96.08 (56 d) | Spinach soil | [28] |
| Tylosin | Burkholderia vietnamiensis | 50–500 | 99 (7 d) | Laboratory soil | [29] | |
| Polypeptides | Polymyxins | Baxillus licheniformis DC-01 | 10 | 92.1 (1d) | Laboratory | [30] |
表选项
目前,少部分有降解抗生素能力的细菌菌株被用于处理含抗生素的土壤或废水,均获得较高的去除率。如章程等从添加抗生素的菠菜土壤中筛选到对泰乐菌素去除率为96.08%的无色杆菌,并将其应用于盆栽土壤中,发现泰乐菌素的残留率在20%以下,表明无色杆菌可以有效促进土壤中泰乐菌素的去除[28];Shao等探究了不同条件下嗜铬菌KSS10对土霉素(Oxytetracycline,OTC)的生物降解特性,发现KSS10菌株在96 h内对OTC的转化率为63.33%。随后对菌株KSS10进行生物固定化并联合生物膜反应器处理合成的水产养殖废水,OTC的清除率约为76.42%[21]。目前,利用抗生素特异性降解细菌菌株处理环境中残留抗生素的应用实例不多,其主要原因可能是在实际应用中存在耐药基因扩散的风险。
1.1.2 降解抗生素的真菌真菌是抗生素的来源之一,同时一些真菌已被证明可以分解抗生素类药物。真菌对高浓度污染物的耐受性比细菌更强,能够降解多种难降解的化合物。另外,真菌细胞内含有细胞色素P450复合物,能够像哺乳动物细胞一样代谢抗生素,使其成为消除环境中残留抗生素的强有力候选者[2, 46](表 2)。
表 2 近十年文献中的抗生素特异性降解真菌Table 2 Antibiotic-degrading fungi reported in recent 10 years
| Antibiotics | Fungus | Concentration (mg/L) | Degradation enzyme | Removal rate (%) | References | |
| Sulfonamides | Sulfamethazine | Trametes versicolor ATCC42530 | 9 | Lac, P450 | 100 (20 h) | [32] |
| Sulfapyridine | Trametes versicolor | 10 | Lac, P450 | 100 (2 d) | [33] | |
| Sulfathiazole | Trametes versicolor | 10 | Lac, P450 | 100 (2 d) | [33] | |
| Sulfamethazine | Phanerochaete chrysosporium | 10–30 | Lac | 53 (1 d) | [34] | |
| Sulfadiazine | Fusarium solani KS256 | 1.5 | —— | 18.53 (7 d) | [35] | |
| Sulfamethoxazole | Phanerochaete Chrysosporium | 10 | Lac, P450, Mnp | 100 (2 d) | [36] | |
| Sulfamethoxazole | Pycnoporus sanguineus | 10 | Lac, P450, Mnp | 85 (30 d) | [36] | |
| Sulfamethoxazole | Trametes versicolor ATCC42530 | 10 | —— | 94 (30 d) | [37] | |
| Sulfamethoxazole | Bjerkandera adusta ATCC28314 | 10 | —— | 94 (30 d) | [37] | |
| Tetracyclines | Tetracycline | Phanerochaete chrysosporium | 50 | Mnp | 72.5 (4 h) | [38] |
| Oxytetracycline | Phanerochaete chrysosporium | 50 | Mnp | 84.3 (4 h) | [38] | |
| Oxytetracycline | Aspergillus sp. Y7 | 200 | —— | 31.86 (7 d) | [39] | |
| Tetracycline | Phanerochaete chrysosporium | 10 | Lip, Mnp | 80 (3 d) | [40] | |
| Tetracycline | Trametes versicolor ATCC 42530 | 2 | —— | 92 (30 d) | [41] | |
| Tetracycline | Bjerkandera adusta ATCC 28314 | 2 | —— | 92 (30 d) | [41] | |
| Oxytetracycline | Trichoderma Harzianum | 250 | —— | 92 (21 d) | [42] | |
| Oxytetracycline | Trichoderma deliquescens | 250 | —— | 85 (21 d) | [42] | |
| Oxytetracycline | Penicillium crustosum | 250 | —— | 83 (21 d) | [42] | |
| Oxytetracycline | Rhodotorula mucilaginosa | 250 | —— | 73 (21 d) | [42] | |
| Oxytetracycline | Talaromyces atroroseus | 250 | —— | 72 (21 d) | [42] | |
| Oxytetracycline | Penicillium janthinellum KS272 | 1.5 | —— | 40.29 (7 d) | [35] | |
| Fluoroquinolones | Ciprofloxacin | Phanerochaete chrysosporium | 2 | Lac, P450 | 90 (7 d) | [43] |
| Norfloxacin | Phanerochaete chrysosporium | 2 | Lac, P450 | 90 (7 d) | [43] | |
| Norfloxacin | Irpex lacteus | 10 | Mnp | 100 (14 d) | [44] | |
| Norfloxacin | Trametes versicolor | 10 | Mnp | 100 (14 d) | [44] | |
| Ofloxacin | Irpex lacteus | 10 | Mnp | 100 (14 d) | [44] | |
| Ofloxacin | Trametes versicolor | 10 | Mnp | 100 (14 d) | [44] | |
| Ciprofloxacin | Irpex lacteus | 10 | Mnp | 100 (14 d) | [44] | |
| Ciprofloxacin | Trametes versicolor | 10 | Mnp | 100 (14 d) | [44] | |
| Ciprofloxacin | Phanerochaete chrysosporium | 10 | Lac, P450 | 98 (2 d) | [36] | |
| Ciprofloxacin | Pycnoporus sanguineus | 10 | Lac, P450 | 98 (2 d) | [36] | |
| Norfloxacin | Phanerochaete chrysosporium | 10 | Lac, P450 | 97 (2 d) | [36] | |
| Norfloxacin | Pycnoporus sanguineus | 10 | Lac, P450 | 97 (2 d) | [36] | |
| Norfloxacin | Penicillium janthinellum KS272 | 1.5 | —— | 10.49 (7 d) | [35] | |
| Beta-lactam | Oxacillin | Leptosphaerulina sp. | 16 | Lac, Mnp, Lip | 100 (6 d) | [41] |
| Cloxacillin | Leptosphaerulina sp. | 17.5 | Lac, Mnp, Lip | 100 (6 d) | [41] | |
| Dicloxacillin | Leptosphaerulina sp. | 19 | Lac, Mnp, Lip | 100 (8 d) | [41] | |
| Cephadroxyl | Leptosphaerulina sp. CECT20913 | 15.2 | Lac, Mnp | 100 (15 d) | [45] | |
| Cephadroxyl | Trametes versicolor ATCC 42530 | 6 | Lac, Mnp | 100 (15 d) | [45] | |
| Macrolipids | Erythromucin | Bjerkandera adusta ATCC28314 | 1.5 | —— | 85 (30 d) | [28] |
表选项
大量的研究表明通过筛选降解抗生素的真菌来去除环境中残留的抗生素是可行的。卿纯等探究了黄孢原毛平革菌应用于四环素(Tetracycline, TC)模拟废水,发现在72 h内该菌株对10 mg/L的TC去除率高达80%[40];Copete-Pertuz等将从哥伦比亚麦德林山谷中分离得到的小光壳属真菌Leptosphaerulia sp.用于处理实验规模的医疗废水,第6天未检测到抗生素的存在,且降解产物无毒害,无抗菌效果[41];崔辉将曲霉属真菌Y-7接种到含有OTC (50 mg/kg)的土壤中,对OTC的降解率可达到30.63%[39];Aydin发现分离自活性污泥中的真菌组合变色栓菌和烟管菌对红霉素(Erythromycin,E)、磺胺甲恶唑(Sulfamethoxazole,SMX)和TC组成的混合抗生素的降解效率在85%–94%之间[37];Lucas等将变色栓菌ATCC42530用于处理废水,对7种不同类型(SAs、TCs和FQ等)的47种抗生素的去除率达到77%,远高于传统的处理方法[47]。
酵母作为一种单细胞真菌,在环境抗生素污染修复方面也具有潜在的价值。Selvi等从制药废水中分离得到一株假丝酵母菌Candida sp. SMN04,它可以利用头孢地尼(250 mg/L)作为唯一碳源生长,6 d内的降解率达到84%[48];冯福鑫等发现,在适当的条件下酵母菌XPY-10在7 d内对初始浓度为600 mg/L的TC去除率为83.63%[49]。以上的研究均说明酵母菌具有高效降解各类抗生素的能力。
菌株的抗生素降解能力受到抗生素种类、菌株类型、碳源、氮源、温度、废水组分等多种因素的影响。为促进抗生素降解菌在实际环境废水中的应用,需要关注以下几点:1)不同抗生素的微生物降解率存在差异。2)不同的菌株类型对不同的抗生素降解效果存在显著差异。3)当环境中的抗生素浓度较低时,作为碳源不足以促进菌群生长,抗生素的降解效率将大大降低。如外加碳源可以有效增强无色杆菌的能量利用效率,提高对磺胺甲恶唑的降解能力[50]。4)菌株在不同的温度条件下对抗生素的降解率具有显著差异[13]。Zheng等长期监控发现,夏季抗生素降解率远高于冬季[51]。5)废水中金属离子的存在会影响氨氧化菌等降解菌还原抗生素的能力[52]。6)抗生素代谢的中间产物如果具有抑菌性会降低菌株的抗生素去除率[10, 23]。7)多株具有降解能力的菌株共培养可以增强对抗生素的降解[53]。
1.2 抗生素的微生物降解途径、基因和降解酶1.2.1 抗生素的微生物降解途径微生物对抗生素的降解过程比较复杂,不同种类的抗生素由于结构不同,降解途径存在显著的差异。总的来说,微生物对抗生素降解途径的主要反应包括:羟基化、乙酰化、硝基化、氧化作用、取代作用等。近年来,有关SAs和TCs的微生物降解机理的报道较多,我们选取了这两类中研究较为透彻的抗生素进行总结。
SMX是SAs中最常用的抗生素之一。其降解主要涉及3个过程:1)主链S-N键断裂;2)异恶唑环裂解;3)发生羟基化、乙酰化或硝基化修饰[54] (图 1)。但在有氧和厌氧条件下,SMX的微生物降解产物存在差异。有氧条件下,SMX作为代谢基质被菌株降解时,途径A产生中间产物3-氨基-5-甲基异恶唑和4-氨基苯磺酸盐或4-苯胺,最终生成产物1, 2, 4-三羟基苯与3-氨基异恶唑;而途径B产生3-氨基-5-甲基异恶唑和4-苯胺,最终生成产物磺胺、苯胺及3-氨基异恶唑[53] (图 1A, B)。最近研究显示,厌氧条件下,SMX有两条降解通路:1) SMX先发生羟基化作用,随后异恶唑环破裂;2)降解菌先攻击异恶唑环,异恶唑环破裂后,形成一个不稳定的自由基阴离子(SMX–),随后发生氢化作用(图 1C)最终形成3-氨基异恶唑[32, 55-56],但是具体的反应步骤有待进一步研究。
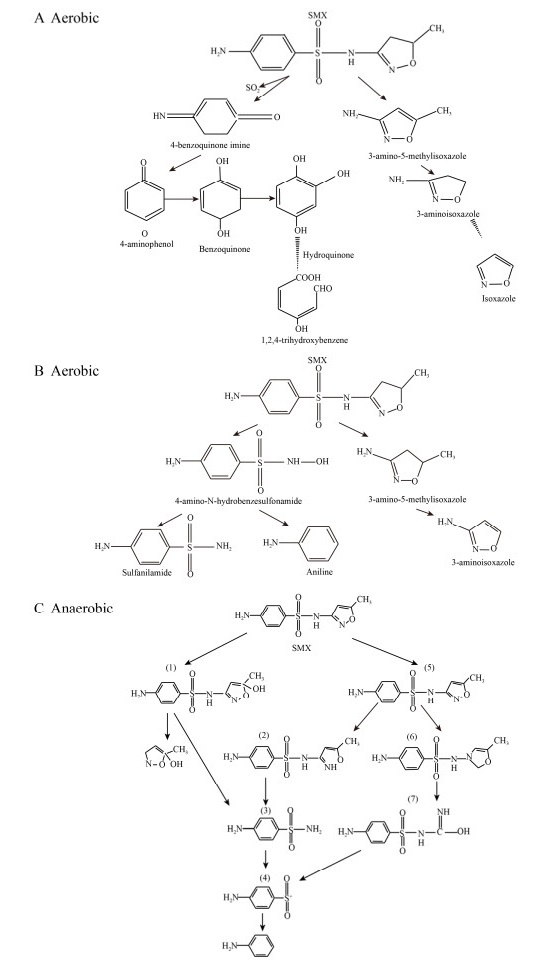 |
| 图 1 SMX的微生物降解途径[53, 55-56] Fig. 1 Microbial degradation pathways of SMX[53, 55-56].(A–B) Biodegradation pathways of SMX under aerobic conditions. (C) Biodegradation pathway of SMX under anaerobic conditions. |
| 图选项 |
OTCs的微生物降解通路目前还不是十分明确,推测可能涉及取代反应、苯环破裂、氧化反应、去羟基化作用。图 2表示了OTC的微生物降解过程:OTCs首先发生了胺基化,形成4-差向土霉素(EOTC);随后发生去羟基化反应形成2-乙酰基-2-去酰胺土霉素(ADOTC),然后氧化形成两种不同构型的产物α-apo-OTC和β-apo-OTC,最终氧化形成3-羟基环己酮[57-58]。
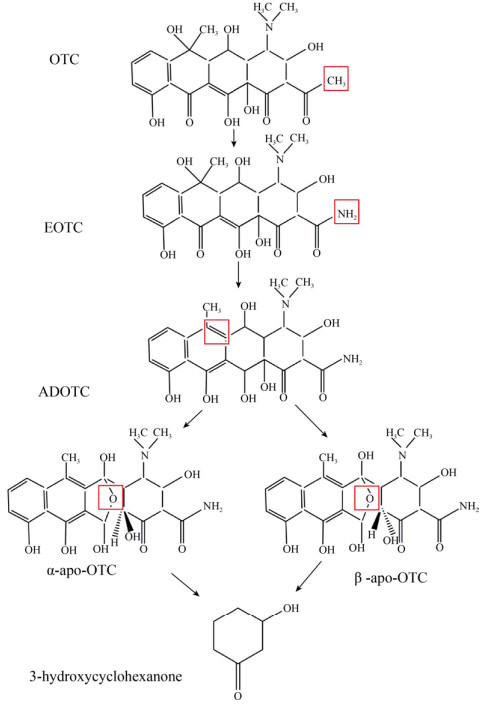 |
| 图 2 土霉素的微生物降解途径[48, 59](土霉素通过胺基化、去羧基化和氧化反应,最终形成产物3-羟基环己酮) Fig. 2 Microbial degradation pathway of OTC. OTC can be converted into 3-hydroxycyclohexanone by amination, decarboxylation and oxidation[48, 59]. |
| 图选项 |
1.2.2 基因和降解酶目前,关于抗生素降解基因的研究主要来自抗生素的耐药菌,这些基因编码的降解酶包括氨基糖类修饰酶、β-内酰胺酶、大环内酯类灭活酶等[60]。Richen报道了SadA、SadB与SadC基因参与了磺胺甲恶唑的生物降解过程,其中SadA、SadB基因编码单加氧酶,SadC基因编码FMN还原酶,这些基因广泛存在于磺胺类的降解菌株中[61]。
近年分离获得的具有抗生素降解能力的真菌以白腐真菌为主,它依赖自身的非特异性酶系统来转化或矿化抗生素等有害的异源物质[38, 62]。该酶系统包括胞外的木质素修饰酶木质素过氧化物酶(Lignin peroxidase, Lip)、锰过氧化物酶(Manganese-dependent peroxidase, Mnp)、漆酶(Laccase, Lac)和胞内的细胞色素P450 (Cytochrome P450)。其中,Lac可以利用O2作为电子受体氧化底物;Mnp可以氧化Mn2+转变为Mn3+与有机酸螯合,随后发生氧化反应[46, 62]。
由于木质素修饰酶是非特异性酶,可以降解环境中难降解的污染物,因此近年来有大量关于木质素修饰酶在抗生素修复方面的应用报道。Llorca等应用漆酶降解四环素和红霉素,去除率达到了78%[63]。Ding等利用SAs和TCs容易被漆酶系统氧化、FQ易被土壤吸附的特点,将漆酶与土壤结合起来,对SAs、TCs和FQ进行去除,其去除率在7.5 d后达到100%[64]。Yang等将漆酶固定化形成磁性交联酶聚合体,TC (100 mg/L)的去除率达到了100%[65]。Marco-Urea等在膜反应器中进行漆酶固定化,并联合介质丁香醛去除高达38种抗生素的混合物,24 h后32种抗生素的降解率大于50%。另有研究将漆酶与细菌进行共培养,提高了漆酶对抗生素的去除效率[66]。Lueangjaroenkit等发现,在金属离子存在的条件下,Mnp和Lac有效地灭活TC、多西环素、阿莫西林和环丙沙星等抗生素[67]。
2 分解抗生素的微生物菌群及应用2.1 分解抗生素的微生物菌群抗生素降解菌群主要由变形菌门、拟杆菌门、酸杆菌门、放线菌门、疣微菌门、浮霉菌门的细菌组成。其中,以β-变形菌门最为丰富,主要负责有机物和养分的去除;亚优势菌门是拟杆菌门、酸杆菌门和绿弯菌门。而真菌中子囊菌门最多,占6%以上。抗生素的添加会影响微生物群落的结构组成,但降解不同种类抗生素的微生物群落的结构差异并不是十分明显[68]。Bai等研究表明,抗生素加入污泥后,微生物群落的丰度显著下降,但多样性增加[69]。降解菌群的组成还受到地理位置、温度及氧气含量的影响。在预脱氮池的系统中,α-变形杆菌的丰度受温度影响,而β-变形杆菌、放线菌和氯氧杆菌在微生物种群中的比例相对稳定。在厌氧条件下,硝化螺旋菌属、生丝微菌属、微丝菌属、甲烷丝菌属、亚硝化单胞菌属的丰度更高,相比于好氧环境,各菌属丰度的分布更加均匀,可能更有利于提高抗生素的降解能力[70-71]。
2.2 应用微生物菌群处理抗生素的方法2.2.1 活性污泥法活性污泥法(Activated sludge process, ASP)是国内外处理污水的常用方法。它的主要机制是,好氧细菌分泌胞外酶,将水中的胶质有机物分解为可溶解有机物。可溶解有机物通过渗透作用进入细菌细胞膜,诱导细胞内特异性基因的表达,随后被分解。同时细菌利用有机物分解释放的能量增殖,进一步加强有机物的降解。ASP自1914年至今经历了漫长的发展历程,现如今已成功应用于抗生素废水的处理。
SAs在活性污泥中的去除以降解为主吸附为辅。如Jia等利用硫酸盐还原菌污泥系统去除SMX,发现SMX起初依赖污泥的快速吸附,随后菌株破坏异恶唑环降解SMX[55]。ASP对不同种类SAs的降解效果存在一定的差异。如Yang等研究发现,ASP对SMX、磺胺二甲氧基嘧啶和磺胺甲氧基嘧啶的降解率分别为24%、30%和19%,降解顺序为SMX > 磺胺二甲氧基 > 磺胺甲嗪[56, 72]。
TCs主要通过生物吸附的方式转移到污泥中,微生物降解的贡献极少[73]。其主要原因是TCs是两性化合物,极易与周围的环境发生反应,形成稳定的化合物,失去抑菌能力[74]。另一可能的原因是特异性降解菌属丰度低。如Wang等发现在原污泥中TC的优势降解菌属如希瓦氏菌、芽孢杆菌、假单胞菌、氨氧化菌等丰度低,降解能力弱。当以TC为唯一碳源时,这些菌属转变为优势菌属,TC降解率显著提高[75]。因此,通过增加活性污泥中抗生素特异性降解菌属的比例,可以提高ASP中微生物群落对抗生素的去除能力。
FQ在ASP的去除以吸附为主微生物降解为辅,高浓度的FQ对ASP的菌群会产生抑制作用。高温、好氧、优势菌株的丰度、硝化作用有助于增强菌群对FQ降解能力[60]。Wang等发现,在有氧、高温条件下,通过硝化过程中的共代谢,显著提高了菌群的FQ去除率[76]。Jia等发现厌氧污泥系统中含有对FQ高耐受性的脱硫杆菌属,能长期有效去除制药及医院废水中的FQ[77]。另外,β-lactams在ASP中的去除也以微生物为辅,高浓度的β-lactams会显著抑制微生物的降解能力。通常将ASP与Fenton氧化、微电解等非生物技术联用提高β-lactams的去除率[78-79]。Chen等将头孢菌素C与活性污泥共堆肥,头孢菌素C的降解率仅为6.58%[80]。传统的ASP存在污泥生产量大、成本高、易膨胀等问题,使其实际应用受到一定的限制[60]。目前,已有不少研究将现代工艺与活性污泥法结合起来,在提高污染物降解率的同时,降低废水处理成本。如Sodhi采用膜生物反应器、厌氧消化池、与CAS反应器连接形成活性污泥改良版系统,促进污泥中微生物的富集,污泥产量减少了72%[81];Meerburg等利用高速率活性污泥,增加了微生物群落的丰度,提高了污泥的质量,为各种污水类型的低容量活性污泥处理设施提供了一种可持续的实用选择[82]。
另外菌群、碳源、反应时间及温度也会影响ASP中抗生素的降解效率:1)抗生素可作为降解菌株的碳源或氮源,当外加乙酸盐和硝酸铵进行共代谢时,抗生素的微生物降解率显著提高[83];2)菌群的丰度和种类会影响抗生素的去除率,其中不动杆菌和假单胞菌是污泥中磺胺降解的主要菌属,脱硫杆菌属是FQ的主要菌属[71];3)反应时间也会影响抗生素的去除效果,细菌对磺胺类抗生素的吸附是一个可逆的过程,当反应时间过长,SAs会被重新释放出来;4)温度影响活性污泥中菌群的生长,如Huang等发现SMZ的吸附速率随温度的升高而降低[11, 51];5)污泥龄在5–25 d时,SMZ的去除率也从45%提高到80%。
2.2.2 膜生物反应器法膜生物反应器(Membrane bioreactor,MBR)是活性污泥处理与微滤或超滤技术相结合的产物。与传统活性污泥处理相比,MBR具有出水水质好、污泥消耗低、占地面积小的特点[60]。近年来,还出现了将微滤或超滤等低压膜与活性污泥系统相结合形成的一种高保留率膜生物反应器。目前有3种高保留率膜生物反应器应用于抗生素废水的处理,即渗透膜生物反应器、膜蒸馏生物反应器和纳滤膜生物反应器,对抗生素具有良好的去除效果,但存在膜污染、膜消耗量大的问题[84-85]。
MBR既包括好氧膜生物反应器,也包括厌氧膜生物反应器(Anaerobic membrane bioreactors, AnMBRs)。AnMBRs是近年来出现的一种处理抗生素废水的新技术。它在处理过程中将废水中的有机物转化为富含甲烷的沼气,并可以通过产生的沼气抵消废水处理过程中的能源需求,与传统的活性污泥和好氧膜生物反应器相比具有许多优点[86]。如Huang等在利用AnMBR系统处理含有β-lactams废水的过程中发现头孢曲松、头孢哌酮等抗生素的去除率达到了50%[87]。另外,AnMBRs膜在阻止抗生素和带有抗性基因的微生物从反应器逃逸到环境中起着重要作用。该系统不仅能降解抗生素,有效地控制细菌污染,而且能提高废水的能量回收,是一种很有前途的抗生素废水处理技术[88]。
随着近年来污水排放标准的提高,越来越多的污水处理厂开始对现有的MBR处理工艺进行改进。如Karaolia将MBR与太阳能芬顿法结合,氧化去除SMX、红霉素和克拉霉素,与单独的MBR相比,克拉霉素的去除率显著提升[89]。Cheng等研究发现将MBRs与生物膜载体(如颗粒活性炭、海绵)相结合可以降低膜污染,提高抗生素的去除率[90]。
MBR的性能还受到盐度、菌群、温度等多种因素的影响。盐分积累会干扰MBR膜的生物性能,出现膜污染、微生物对抗生素的去除能力下降等问题[91]。耐盐菌群的增加会维护MBR的性能。Luo等研究发现,在高盐度条件下,大部分菌属受到抑制,耐盐/嗜盐微生物与非嗜盐微生物的比例显著升高,MBR的生物学性能随耐盐菌的增加缓慢恢复,因此可以通过增加耐盐菌或嗜盐菌的量来维持MBR的性能[85]。温度过高或过低对MBR的抗生素去除效率有显著性影响[92]。
2.2.3 堆肥法堆肥法是一种将原生有机质转化为有价值的有机土壤的改良技术,它通过多种微生物的作用,将生物残体、粪便和药渣等进行矿质化、腐殖化和无害化,使得各种复杂的有机养分转化为可溶性养分和腐殖质,可作为去除动物粪便中抗生素的一种有效方法。堆肥法在20世纪初由英国农业学家霍华德提出,主要有好氧堆肥和厌氧堆肥两种类型,在不同类型抗生素的降解中均有应用。好氧堆肥是一种通过酶、微生物和氧气的作用降解有机物的过程[93]。厌氧堆肥则是一个发酵的过程,它利用畜禽粪便产生环境友好型能源(沼气),主要由4个阶段组成,即水解、酸生成、乙酰生成和甲烷生成。
Shi等在粪便中添加4种典型的抗生素进行好氧堆肥,20 d内抗生素的去除率达到了90%以上[94]。Inastrazysch等研究发现厌氧发酵对7种磺胺类药物和甲氧苄啶均有去除效果,而且代谢物的抗菌活性显著降低[95];Spielmeyer等发现通过半连续发酵,磺胺嘧啶、四环素和氯四环素的去除率在14%–89%之间,并且抗生素的存在对甲烷产量没有抑制作用[96]。
堆肥法的抗生素清除率受到堆肥底物、温度等多种因素的影响。(1)共堆肥有利于抗生素的清除[97]。Zhang等将青霉素发酵菌渣与猪粪进行好氧共堆肥后,超过99%的青霉素在堆肥7 d后被清除[97-98];Liu等利用庆大霉素发酵残基和洛伐他汀发酵残基进行室内共堆肥,庆大霉素去除率在90%以上[8]。(2)温度、pH。Huang等研究发现,堆肥的最佳pH值在5.5–8.0之间[99];Ma等发现高温能有效促进堆肥发酵[100]。(3)过少的曝气会导致厌氧环境,而过多的曝气会导致过早冷却,破坏了适宜的高温条件,从而影响分解速率[93, 101]。
2.2.4 生物电化学系统生物电化学系统(Bioelectrochemical systems, BESs)由微生物燃料电池(MFCs)和微生物电解细胞(MECs)两部分组成,是一种将微生物代谢和电化学氧化还原反应结合起来,利用电化学性微生物回收能量的装置,被认为是降解污染物的有效的替代方法,近年被应用到抗生素的降解中[102-103]。
大部分的MFC是由生物阳极和非生物阴极组成的。在非生物阴极中,通常铁氰化钾或氧作为电子受体;在生物阳极中,抗生素作为电子供体和碳源。产电菌和降解菌附着在阳极上,在细胞外聚合物中形成生物膜,负责降低可降解抗生素及其代谢物的电位,无需提供外源能量[102]。
目前,已有不少证据表明BESs系统对抗生素具有良好的去除效果。如MFC对磺胺嘧啶、SMX等SAs降解率在85%以上,对TC的降解率能达到99%,对氯霉素也具有良好的去除效果[102, 104]。与其他技术相比,BESs具有以下优势:(1)运行成本低[106];(2)抗生素去除效果好[105-106];(3)可以与其他技术如人工湿地联用,降解不同类型的抗生素,但它的降解能力也受到盐度、固相以及金属的影响[107-108]。
2.2.5 微藻的光解藻类是一类结构类似于细菌的真核生物。微藻在水生环境中具有最多的生物量,对污染物的耐受性也高于细菌,并且具有良好的有机物去除能力[109]。微藻广泛应用于各种新型污染物废水的处理,近年也出现了利用微藻去除废水中抗生素的报道。如Liu等将铜绿微囊藻置于50 μg/L–1 ng/L螺旋霉素和阿莫西林水溶液中培养7 d,降解了12.5%–32.9%的螺旋霉素和30.5%–33.6%的阿莫西林,表明蓝藻细胞具有一定的抗生素去除能力[110]。Hom-Diaz等采用光藻反应器处理实际废水,可高效去除废水中的环丙沙星(2 mg/L)[111]。Yu等研究发现,在海藻处理6 h后,未处理的头孢噻啶残留率为96.93%,处理后的头孢噻啶的残留率仅为7.35%[112]。有研究发现光照强度、CO2浓度有利于藻类生长和抗生素的去除[113]。微藻对抗生素的处理主要由3个步骤组成:1)快速吸附,藻类具有较大的表面积和体积比,其细胞壁带有负电荷,因此能吸附大量的有机物;2)缓慢的细胞壁传输进入微藻细胞;3)光解作用,将光电子的光激发与微藻的光催化耦合,在抗生素光解过程中促进了光电子的传递[109]。
近年来,也有研究将微藻与活性污泥联用来处理抗生素废水。Guo等利用藻类和活性污泥联合系统去除头孢菌素,14 d内单活性污泥的去除率为46.3%,联合去除效果达到了97.91%[114]。
3 总结与展望抗生素作为一类新型污染物,给水体、土壤造成的污染成为全球面临的重大环境问题。在自然环境中,生物降解是天然存在于生态系统中的有机物降解途径。相较于传统修复方法,其成本低、效能高、适用范围广,是一种具有前景的消除环境抗生素残留的生物修复法。目前,关于不同种类抗生素微生物代谢通路的研究已有大量文献报道。但现有研究主要集中于抗生素特异性降解菌株的筛选与降解产物的分析,对降解过程所涉及的基因及产物、降解机理的研究及特异性菌株的实际应用很少。为了能使微生物修复法得到更广泛的应用,今后的环境抗生素污染的微生物修复研究可以从以下方面开展:1)探究在自然和实验条件下,抗生素特异性降解菌株生存和降解能力的差异。2)筛选对特定类别多种抗生素具有极强降解能力的菌株。3)加强对抗生素生物降解机制的研究,挖掘参与抗生素降解过程的关键基因元件、降解酶,加速对抗生素降解酶制剂的研究。4)应用合成生物学方法构建和改造工程菌株,提高菌株对多种抗生素的分解能力[31]。5)抗生素降解菌株、菌群与非生物修复技术联用,并优化组合工艺,提高抗生素的降解效率。
参考文献
| [1] | Tan SY, Tatsumura Y. Alexander fleming (1881-1955): discoverer of penicillin. Singapore Med J, 2015, 56(7): 366-367. DOI:10.11622/smedj.2015105 |
| [2] | Kumar M, Jaiswal S, Sodhi KK, et al. Antibiotics bioremediation: perspectives on its ecotoxicity and resistance. Environ Int, 2019, 124: 448-461. |
| [3] | Wang Z, Zhang H, Han J, et al. Deadly sins of antibiotic abuse in China. Infect Control Hosp Epidemiol, 2017, 38(6): 758-759. DOI:10.1017/ice.2017.60 |
| [4] | Reis PJM, Reis AC, Ricken B, et al. Biodegradation of sulfamethoxazole and other sulfonamides by Achromobacter denitrificans PR1. J Hazard Mater, 2014, 280: 741-749. DOI:10.1016/j.jhazmat.2014.08.039 |
| [5] | Li N, Sheng GP, Lu YZ, et al. Removal of antibiotic resistance genes from wastewater treatment plant effluent by coagulation. Water Res, 2017, 111: 204-212. DOI:10.1016/j.watres.2017.01.010 |
| [6] | Sharma VK, Johnson N, Cizmas L, et al. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere, 2016, 150: 702-714. DOI:10.1016/j.chemosphere.2015.12.084 |
| [7] | Luo Y, Huang B, Jin Y, et al. Research progress in the degradation of antibiotics wastewater treatment. Chem Ind Eng Prog, 2014, 33(9): 2471-2477 (in Chinese). 罗玉, 黄斌, 金玉, 等. 污水中抗生素的处理方法研究进展. 化工进展, 2014, 33(9): 2471-2477. |
| [8] | Liu YW, Feng Y, Cheng DM, et al. Gentamicin degradation and changes in fungal diversity and physicochemical properties during composting of gentamicin production residue. Bioresour Technol, 2017, 244: 905-912. DOI:10.1016/j.biortech.2017.08.057 |
| [9] | Ricken B, Fellmann O, Kohler HPE, et al. Degradation of sulfonamide antibiotics by Microbacterium sp. strain BR1-elucidating the downstream pathway. N Biotechnol, 2015, 32(6): 710-715. DOI:10.1016/j.nbt.2015.03.005 |
| [10] | Kassotaki E, Buttiglieri G, Ferrando-Climent L, et al. Enhanced sulfamethoxazole degradation through ammonia oxidizing bacteria co-metabolism and fate of transformation products. Water Res, 2016, 94: 111-119. DOI:10.1016/j.watres.2016.02.022 |
| [11] | Huang MH, Tian SX, Chen DH, et al. Removal of sulfamethazine antibiotics by aerobic sludge and an isolated Achromobacter sp. S-3.. J Environ Sci, 2012, 24(9): 1594-1599. DOI:10.1016/S1001-0742(11)60973-X |
| [12] | Reis PJM, Homem V, Alves A, et al. Insights on sulfamethoxazole bio-transformation by environmental Proteobacteria isolates. J Hazard Mater, 2018, 358: 310-318. DOI:10.1016/j.jhazmat.2018.07.012 |
| [13] | Wang SZ, Wang JL. Biodegradation and metabolic pathway of sulfamethoxazole by a novel strain Acinetobacter sp. Appl Microbiol Biotechnol, 2018, 102(1): 425-432. |
| [14] | Zhang JY, Peng XX, Jia XS. Isolation and characterization of high efficiency sulfamethazine- degrading bacterium strain J2. Acta Sci Circumstant, 2019, 39(09): 2919-2927 (in Chinese). 张珈瑜, 彭星星, 贾晓珊. 磺胺二甲基嘧啶(SM2)高效降解菌J2的分离筛选及降解特性研究. 环境科学学报, 2019, 39(09): 2919-2927. |
| [15] | Mulla SI, Hu AY, Sun Q, et al. Biodegradation of sulfamethoxazole in bacteria from three different origins. J Environ Manage, 2018, 206: 93-102. DOI:10.1016/j.jenvman.2017.10.029 |
| [16] | Zhang XY, Cai TJ, Xu XP. Isolation and identification of a tetracycline-degrading bacterium and optimizing condition for tetracycline degradation. Biotechnol Bull, 2015, 31(1): 173-180 (in Chinese). 张欣阳, 蔡婷静, 许旭萍. 一株高效四环素降解菌的分离鉴定及其降解性能研究. 生物技术通报, 2015, 31(1): 173-180. |
| [17] | Leng YF. Characteristics and mechanism of tetracycline degradation by microorganism[D]. Wuhan: China University of Geosciences, 2017 (in Chinese). 冷一非.微生物降解四环素特性及降解机理研究[D].武汉: 中国地质大学, 2017. http://cdmd.cnki.com.cn/Article/CDMD-10491-1017740208.htm |
| [18] | Wu XL, Wu XY, Li JK, et al. Isolation and degradation characteristics of a efficient tetracycline-degrading strain. Biotechnol Bull, 2018, 34(5): 172-178 (in Chinese). 吴学玲, 吴晓燕, 李交昆, 等. 一株四环素高效降解菌的分离及降解特性. 生物技术通报, 2018, 34(5): 172-178. |
| [19] | Shen FY, Sun MM, Jiao JG, et al. Effects and response process of tetracycline on bioremediation of pyrene-contaminated soil. Soils, 2016, 48(5): 954-963 (in Chinese). 沈方圆, 孙明明, 焦加国, 等. 四环素对芘污染农田土壤微生物修复的影响及响应过程. 土壤, 2016, 48(5): 954-963. |
| [20] | Meng YH, Feng Y, Li XF, et al. Isolation of an oxytetracycline-degrading bacterial strain and its biodegradation characteristics. J Plant Nutr Fert, 2018, 24(3): 720-727 (in Chinese). 孟应宏, 冯瑶, 黎晓峰, 等. 土霉素降解菌筛选及降解特性研究. 植物营养与肥料学报, 2018, 24(3): 720-727. |
| [21] | Shao SC, Hu YY, Cheng JH, et al. Degradation of oxytetracycline (OTC) and nitrogen conversion characteristics using a novel strain. Chem Eng J, 2018, 354: 758-766. DOI:10.1016/j.cej.2018.08.032 |
| [22] | Cheng J, Du HL, Zhang TB, et al. Isolation and identification of tetracyclines degrading bacteria. J Nucl Agric Sci, 2017, 31(5): 884-888 (in Chinese). 成洁, 杜慧玲, 张天宝, 等. 四环素类抗生素降解菌的分离与鉴定. 核农学报, 2017, 31(5): 884-888. |
| [23] | Pan LJ, Li J, Li CX, et al. Study of ciprofloxacin biodegradation by a Thermus sp. isolated from pharmaceutical sludge. J Hazard Mater, 2018, 343: 59-67. DOI:10.1016/j.jhazmat.2017.09.009 |
| [24] | Fu BM, Chen LW, Cai TM, et al. Isolation and characterization of norfloxacin-degrading bacterium strain NOR-36. Acta Sci Circumstant, 2017, 37(2): 576-584 (in Chinese). 付泊明, 陈立伟, 蔡天明, 等. 诺氟沙星降解菌NOR-36的分离筛选及降解特性研究. 环境科学学报, 2017, 37(2): 576-584. |
| [25] | Maia AS, Tiritan ME, Castro PML. Enantioselective degradation of ofloxacin and levofloxacin by the bacterial strains Labrys portucalensis F11 and Rhodococcus sp. FP1. Ecotoxicol Environ Saf, 2018, 155: 144-151. DOI:10.1016/j.ecoenv.2018.02.067 |
| [26] | Lin BK, Lyu J, Lyu XJ, et al. Characterization of cefalexin degradation capabilities of two Pseudomonas strains isolated from activated sludge. J Hazard Mater, 2015, 282: 158-164. DOI:10.1016/j.jhazmat.2014.06.080 |
| [27] | Liu H, Yang YK, Ge YH, et al. Interaction between common antibiotics and a Shewanella strain isolated from an enhanced biological phosphorus removal activated sludge system. Bioresour Technol, 2016, 222: 114-122. DOI:10.1016/j.biortech.2016.09.096 |
| [28] | Zhang C, Feng Y, Liu YW, et al. The degradation of typical antibiotics and their effects on soil bacterial diversity in spinach soil. Sci Agric Sin, 2018, 51(19): 3736-3749 (in Chinese). 章程, 冯瑶, 刘元望, 等. 菠菜土壤中典型抗生素的微生物降解及细菌多样性. 中国农业科学, 2018, 51(19): 3736-3749. DOI:10.3864/j.issn.0578-1752.2018.19.011 |
| [29] | Wang Y, Ma YL, Ma L, et al. Study on microbial degradation pathway and products of tylosin residue in pharmaceutical waste. Acta Sci Circumstant, 2015, 35(2): 491-498 (in Chinese). 王艳, 马玉龙, 马琳, 等. 泰乐菌素的微生物降解途径及其降解产物研究. 环境科学学报, 2015, 35(2): 491-498. |
| [30] | Wang G, Yu ZL, Qiu JP. Screening of polymyxin-degrading bacteria and optimization of its culture conditions. Bull Ferment Sci Technol, 2018, 47(1): 21-26 (in Chinese). 王刚, 余志良, 裘娟萍. 多粘菌素E降解酶产生菌的筛选及其产酶条件的研究. 发酵科技通讯, 2018, 47(1): 21-26. |
| [31] | Tang HZ, Wang WW, Zhang LG, et al. Application of synthetic biology in environmental remediation. Chin J Biotechnol, 2017, 33(3): 506-515 (in Chinese). 唐鸿志, 王伟伟, 张莉鸽, 等. 合成生物学在环境修复中的应用. 生物工程学报, 2017, 33(3): 506-515. |
| [32] | García-Galán MJ, Rodríguez-Rodríguez CE, Vicent T, et al. Biodegradation of sulfamethazine by Trametes versicolor: removal from sewage sludge and identification of intermediate products by UPLC-QqTOF-MS. Sci Total Environ, 2011, 409(24): 5505-5512. DOI:10.1016/j.scitotenv.2011.08.022 |
| [33] | Rodríguez-Rodríguez CE, García-Galán MJ, Blánquez P, et al. Continuous degradation of a mixture of sulfonamides by Trametes versicolor and identification of metabolites from sulfapyridine and sulfathiazole. J Hazard Mater, 2012, 213-214: 347-354. DOI:10.1016/j.jhazmat.2012.02.008 |
| [34] | Guo XL, Zhu ZW, Li HL. Biodegradation of sulfamethoxazole by Phanerochaete chrysosporium. J Mol Liq, 2014, 198: 169-172. DOI:10.1016/j.molliq.2014.06.017 |
| [35] | Wang QF, Zhu PL, Xia ZM, et al. Screening and degradation properties of three kinds of agricultural antibiotics degrading fungi. J Agric Resourc Environ, 2018, 35(6): 533-539 (in Chinese). 王强锋, 朱彭玲, 夏中梅, 等. 三种农用抗生素降解真菌的筛选及其降解性能. 农业资源与环境学报, 2018, 35(6): 533-539. |
| [36] | Gao N, Liu CX, Xu QM, et al. Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere, 2018, 195: 146-155. DOI:10.1016/j.chemosphere.2017.12.062 |
| [37] | Aydin S. Enhanced biodegradation of antibiotic combinations via the sequential treatment of the sludge resulting from pharmaceutical wastewater treatment using white-rot fungi Trametes versicolor and Bjerkandera adusta. Appl Microbiol Biotechnol, 2016, 100(14): 6491-6499. DOI:10.1007/s00253-016-7473-0 |
| [38] | Wen XH, Jia YN, Li JX. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from Phanerochaete chrysosporium. J Hazard Mater, 2010, 177(1/3): 924-928. |
| [39] | Zhai H. Isolation and identification of oxytetracycline-degrading strain and its use in bioremediation simulation of contaminated soil[D]. Yangling: Northwest A & F University, 2016 (in Chinese). 翟辉.土霉素降解菌的筛选、鉴定及其在污染土壤中的修复模拟[D].杨凌: 西北农林科技大学, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10712-1016157555.htm |
| [40] | Qing C, Shang C, Zhou YH, et al. Study on the biodegradation of tetracycline wastewater by Phanerochaete chrysosporium. Environ Pollut Control, 2018, 40(9): 1023-1026, 1067 (in Chinese). 卿纯, 尚翠, 周亦航, 等. 黄孢原毛平革菌对四环素废水的降解研究. 环境污染与防治, 2018, 40(9): 1023-1026, 1067. |
| [41] | Copete-Pertuz LS, Plácido J, Serna-Galvis EA, et al. Elimination of isoxazolyl-penicillins antibiotics in waters by the ligninolytic native Colombian strain Leptosphaerulina sp. considerations on biodegradation process and antimicrobial activity removal. Sci Total Environ, 2018, 630: 1195-1204. DOI:10.1016/j.scitotenv.2018.02.244 |
| [42] | Ahumada-Rudolph R, Novoa V, Sáez K, et al. Marine fungi isolated from Chilean fjord sediments can degrade oxytetracycline. Environ Monit Assess, 2016, 188(8): 468. DOI:10.1007/s10661-016-5475-0 |
| [43] | Prieto A, M?der M, Rodil R, et al. Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour Technol, 2011, 102(23): 10987-10995. DOI:10.1016/j.biortech.2011.08.055 |
| [44] | ?van?arová M, Moeder M, Filipová A, et al. Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi - Metabolites, enzymes and residual antibacterial activity. Chemosphere, 2015, 136: 311-320. DOI:10.1016/j.chemosphere.2014.12.012 |
| [45] | Pérez-Grisales MS, Castrillón-Tobón M, Copete-Pertuz LS, et al. Biotransformation of the antibiotic agent cephadroxyl and the synthetic dye Reactive Black 5 by Leptosphaerulina sp. immobilised on Luffa (Luffa cylindrica) sponge. Biocatal Agric Biotechnol, 2019, 18: 101051. DOI:10.1016/j.bcab.2019.101051 |
| [46] | Yu M, Ruan XW, Tan LQ, et al. Study on impact factors of manganese-dependent peroxidase produced by white rot fungi. Chem Bioeng, 2014, 31(11): 40-44 (in Chinese). 余梅, 阮小文, 谭丽泉, 等. 白腐真菌产锰过氧化物酶的研究. 化学与生物工程, 2014, 31(11): 40-44. DOI:10.3969/j.issn.0438-1157.2014.12.10 |
| [47] | Lucas D, Badia-Fabregat M, Vicent T, et al. Fungal treatment for the removal of antibiotics and antibiotic resistance genes in veterinary hospital wastewater. Chemosphere, 2016, 152: 301-308. DOI:10.1016/j.chemosphere.2016.02.113 |
| [48] | Selvi A, Das D, Das N. Potentiality of yeast Candida sp. SMN04 for degradation of cefdinir, a cephalosporin antibiotic: kinetics, enzyme analysis and biodegradation pathway. Environ Technol, 2015, 36(24): 3312-3124. |
| [49] | Feng FX, Xu XP, Cheng QX, et al. Degradation characteristics of tetracycline hydrochloride by Trichosporon mycotoxinivorans XPY-10. Chin J Environ Eng, 2013, 7(12): 4779-4785 (in Chinese). 冯福鑫, 许旭萍, 程群星, 等. 四环素高效降解酵母菌Trichosporon mycotoxinivorans XPY-10降解特性. 环境工程学报, 2013, 7(12): 4779-4785. |
| [50] | Nguyen PY, Carvalho G, Reis AC, et al. Impact of biogenic substrates on sulfamethoxazole biodegradation kinetics by Achromobacter denitrificans strain PR1. Biodegradation, 2017, 28(2/3): 205-217. |
| [51] | Zheng WL, Wen XH, Zhang B, et al. Selective effect and elimination of antibiotics in membrane bioreactor of urban wastewater treatment plant. Sci Total Environ, 2019, 646: 1293-1303. DOI:10.1016/j.scitotenv.2018.07.400 |
| [52] | Liu H, Yang YK, Sun HF, et al. Effect of tetracycline on microbial community structure associated with enhanced biological N & P removal in sequencing batch reactor. Bioresour Technol, 2018, 256: 414-420. DOI:10.1016/j.biortech.2018.02.051 |
| [53] | Wang JL, Wang SZ. Microbial degradation of sulfamethoxazole in the environment. Appl Microbiol Biotechnol, 2018, 102(8): 3573-3582. DOI:10.1007/s00253-018-8845-4 |
| [54] | Chen JF, Xie SG. Overview of sulfonamide biodegradation and the relevant pathways and microorganisms. Sci Total Environ, 2018, 640-641: 1465-1477. DOI:10.1016/j.scitotenv.2018.06.016 |
| [55] | Jia YY, Khanal SK, Zhang HQ, et al. Sulfamethoxazole degradation in anaerobic sulfate-reducing bacteria sludge system. Water Res, 2017, 119: 12-20. DOI:10.1016/j.watres.2017.04.040 |
| [56] | Yang CW, Hsiao WC, Chang BV. Biodegradation of sulfonamide antibiotics in sludge. Chemosphere, 2016, 150: 559-565. DOI:10.1016/j.chemosphere.2016.02.064 |
| [57] | Migliore L, Fiori M, Spadoni A, et al. Biodegradation of oxytetracycline by Pleurotus ostreatus mycelium: a mycoremediation technique. J Hazard Mater, 2012, 215-216: 227-232. DOI:10.1016/j.jhazmat.2012.02.056 |
| [58] | Li ZJ, Qi WN, Feng Y, et al. Degradation mechanisms of oxytetracycline in the environment. J Integr Agric, 2018, 17: 60345-60347. |
| [59] | Wang J, Zhou BY, Ge RJ, et al. Degradation characterization and pathway analysis of chlortetracycline and oxytetracycline in a microbial fuel cell. RSC Adv, 2018, 8(50): 28613-28624. DOI:10.1039/C8RA04904A |
| [60] | Liu YW, Li ZJ, Feng Y, et al. Research progress in microbial degradation of antibiotics. J Agro-Environ Sci, 2016, 35(2): 212-224 (in Chinese). 刘元望, 李兆君, 冯瑶, 等. 微生物降解抗生素的研究进展. 农业环境科学学报, 2016, 35(2): 212-224. |
| [61] | Ricken B, Kolvenbach BA, Bergesch C, et al. FMNH2-dependent monooxygenases initiate catabolism of sulfonamides in Microbacterium sp. strain BR1 subsisting on sulfonamide antibiotics. Sci Rep, 2017, 7: 15783. DOI:10.1038/s41598-017-16132-8 |
| [62] | Falade AO, Nwodo UU, Iweriebor BC, et al. Lignin peroxidase functionalities and prospective applications. MicrobiologyOpen, 2017, 6(1): e00394. DOI:10.1002/mbo3.394 |
| [63] | Llorca M, Rodríguez-Mozaz S, Couillerot O, et al. Identification of new transformation products during enzymatic treatment of tetracycline and erythromycin antibiotics at laboratory scale by an on-line turbulent flow liquid-chromatography coupled to a high resolution mass spectrometer LTQ-Orbitrap. Chemosphere, 2015, 119: 90-98. DOI:10.1016/j.chemosphere.2014.05.072 |
| [64] | Ding HJ, Wu YX, Zou BC, et al. Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J Hazard Mater, 2016, 307: 350-358. DOI:10.1016/j.jhazmat.2015.12.062 |
| [65] | Yang J, Lin YH, Yang XD, et al. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J Hazard Mater, 2017, 322: 525-531. DOI:10.1016/j.jhazmat.2016.10.019 |
| [66] | Li X, Xu QM, Cheng JS, et al. Improving the bioremoval of sulfamethoxazole and alleviating cytotoxicity of its biotransformation by laccase producing system under coculture of Pycnoporus sanguineus and Alcaligenes faecalis. Bioresour Technol, 2016, 220: 333-340. DOI:10.1016/j.biortech.2016.08.088 |
| [67] | Lueangjaroenkit P, Teerapatsakul C, Sakka K, et al. Two manganese peroxidases and a laccase of Trametes polyzona KU-RNW027 with novel properties for dye and pharmaceutical product degradation in redox mediator-free system. Mycobiology, 2019. DOI:10.1080/12298093.2019.1589900 |
| [68] | Cycoń M, Mrozik A, Piotrowska-Seget Z. Antibiotics in the soil environment-degradation and their impact on microbial activity and diversity. Front Microbiol, 2019, 10: 338. DOI:10.3389/fmicb.2019.00338 |
| [69] | Bai Y, Xu R, Wang QP, et al. Sludge anaerobic digestion with high concentrations of tetracyclines and sulfonamides: dynamics of microbial communities and change of antibiotic resistance genes. Bioresour Technol, 2019, 276: 51-59. DOI:10.1016/j.biortech.2018.12.066 |
| [70] | Cydzik-Kwiatkowska A, Zielińska M. Bacterial communities in full-scale wastewater treatment systems. World J Microbiol Biotechnol, 2016, 32(4): 66. DOI:10.1007/s11274-016-2012-9 |
| [71] | Zhao RX, Feng J, Liu J, et al. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res, 2019, 151: 388-402. DOI:10.1016/j.watres.2018.12.034 |
| [72] | Yang SF, Lin CF, Lin AYC, et al. Sorption and biodegradation of sulfonamide antibiotics by activated sludge: experimental assessment using batch data obtained under aerobic conditions. Water Res, 2011, 45(11): 3389-3397. DOI:10.1016/j.watres.2011.03.052 |
| [73] | Huang MH, Yang YD, Chen DH, et al. Removal mechanism of trace oxytetracycline by aerobic sludge. Process Saf Environ Prot, 2012, 90(2): 141-146. DOI:10.1016/j.psep.2011.08.008 |
| [74] | Yang QX, Yang XF, Yan Y, et al. Heterogeneous activation of peroxymonosulfate by different ferromanganese oxides for tetracycline degradation: structure dependence and catalytic mechanism. Chem Eng J, 2018, 348: 263-270. DOI:10.1016/j.cej.2018.04.206 |
| [75] | Wang Q, Li XN, Yang QX, et al. Evolution of microbial community and drug resistance during enrichment of tetracycline-degrading bacteria. Ecotoxicol Environ Saf, 2019, 171: 746-752. DOI:10.1016/j.ecoenv.2019.01.047 |
| [76] | Wang L, Qiang ZM, Li YG, et al. An insight into the removal of fluoroquinolones in activated sludge process: sorption and biodegradation characteristics. J Environ Sci (China), 2017, 56: 263-271. DOI:10.1016/j.jes.2016.10.006 |
| [77] | Jia YY, Khanal SK, Shu HY, et al. Ciprofloxacin degradation in anaerobic sulfate-reducing bacteria (SRB) sludge system: mechanism and pathways. Water Res, 2018, 136: 64-74. DOI:10.1016/j.watres.2018.02.057 |
| [78] | Guo RX, Xie XD, Chen JQ. The degradation of antibiotic amoxicillin in the Fenton-activated sludge combined system. Environ Technol, 2015, 36(7): 844-851. DOI:10.1080/09593330.2014.963696 |
| [79] | Qi YF, He SB, Wu SQ, et al. Utilization of micro-electrolysis, up-flow anaerobic sludge bed, anoxic/oxic-activated sludge process, and biological aerated filter in penicillin G wastewater treatment. Desalin Water Treat, 2015, 55(6): 1480-1487. DOI:10.1080/19443994.2014.926259 |
| [80] | Chen ZQ, Wang Y, Wen QX, et al. Feasibility study of recycling cephalosporin C fermentation dregs using co-composting process with activated sludge as co-substrate. Environ Technol, 2016, 37(17): 2222-2230. DOI:10.1080/09593330.2016.1146340 |
| [81] | Sodhi V, Bansal A, Jha MK. Excess sludge disruption and pollutant removal from tannery effluent by upgraded activated sludge system. Bioresour Technol, 2018, 263: 613-624. |
| [82] | Meerburg FA, Vlaeminck SE, Roume H, et al. High-rate activated sludge communities have a distinctly different structure compared to low-rate sludge communities, and are less sensitive towards environmental and operational variables. Water Res, 2016, 100: 137-145. DOI:10.1016/j.watres.2016.04.076 |
| [83] | Müller E, Schüssler W, Horn H, et al. Aerobic biodegradation of the sulfonamide antibiotic sulfamethoxazole by activated sludge applied as co-substrate and sole carbon and nitrogen source. Chemosphere, 2013, 92(8): 969-978. DOI:10.1016/j.chemosphere.2013.02.070 |
| [84] | Nie YF, Qiang ZM, Ben WW, et al. Removal of endocrine-disrupting chemicals and conventional pollutants in a continuous-operating activated sludge process integrated with ozonation for excess sludge reduction. Chemosphere, 2014, 105: 133-138. DOI:10.1016/j.chemosphere.2014.01.006 |
| [85] | Luo WH, Phan HV, Hai FI, et al. Effects of salinity build-up on the performance and bacterial community structure of a membrane bioreactor. Bioresour Technol, 2016, 200: 305-310. DOI:10.1016/j.biortech.2015.10.043 |
| [86] | Robles á, Ruano MV, Charfi A, et al. A review on anaerobic membrane bioreactors (AnMBRs) focused on modelling and control aspects. Bioresour Technol, 2018, 270: 612-626. DOI:10.1016/j.biortech.2018.09.049 |
| [87] | Huang B, Wang HC, Cui D, et al. Treatment of pharmaceutical wastewater containing β-lactams antibiotics by a pilot-scale anaerobic membrane bioreactor (AnMBR). Chem Eng J, 2018, 341: 238-247. DOI:10.1016/j.cej.2018.01.149 |
| [88] | Song XY, Luo WH, Hai FI, et al. Resource recovery from wastewater by anaerobic membrane bioreactors: opportunities and challenges. Bioresour Technol, 2018, 270: 669-677. DOI:10.1016/j.biortech.2018.09.001 |
| [89] | Karaolia P, Kordatou I, Hapeshi E, et al. Investigation of the potential of a membrane bioreactor followed by solar Fenton oxidation to remove antibiotic-related microcontaminants. Chem Eng J, 2017, 310: 491-502. DOI:10.1016/j.cej.2016.04.113 |
| [90] | Cheng DL, Ngo HH, Guo WS, et al. Anaerobic membrane bioreactors for antibiotic wastewater treatment: performance and membrane fouling issues. Bioresour Technol, 2018, 267: 714-724. DOI:10.1016/j.biortech.2018.07.133 |
| [91] | Aslam M, Charfi A, Lesage G, et al. Membrane bioreactors for wastewater treatment: a review of mechanical cleaning by scouring agents to control membrane fouling. Chem Eng J, 2017, 307: 897-913. DOI:10.1016/j.cej.2016.08.144 |
| [92] | Luo WH, Hai FI, Price WE, et al. High retention membrane bioreactors: challenges and opportunities. Bioresour Technol, 2014, 167: 539-546. DOI:10.1016/j.biortech.2014.06.016 |
| [93] | Chen ZQ, Zhang SH, Wen QX, et al. Effect of aeration rate on composting of penicillin mycelial dreg. J Environ Sci (China), 2015, 37: 172-178. DOI:10.1016/j.jes.2015.03.020 |
| [94] | Shi HL, Wang XC, Li Q, et al. Degradation of typical antibiotics during human feces aerobic composting under different temperatures. Environ Sci Pollut Res, 2016, 23(15): 15076-15087. DOI:10.1007/s11356-016-6664-7 |
| [95] | Spielmeyer A, Breier B, Groi?meier K, et al. Elimination patterns of worldwide used sulfonamides and tetracyclines during anaerobic fermentation. Bioresour Technol, 2015, 193: 307-314. DOI:10.1016/j.biortech.2015.06.081 |
| [96] | Spielmeyer A, Stahl F, Petri MS, et al. Transformation of sulfonamides and tetracyclines during anaerobic fermentation of liquid manure. J Environ Qual, 2017, 46(1): 160-168. |
| [97] | Zhang ZH, Zhao J, Yu CG, et al. Evaluation of aerobic co-composting of penicillin fermentation fungi residue with pig manure on penicillin degradation, microbial population dynamics and composting maturity. Bioresour Technol, 2015, 198: 403-409. DOI:10.1016/j.biortech.2015.09.005 |
| [98] | Zhang SH, Chen ZQ, Wen QX, et al. Assessing the stability in composting of penicillin mycelial dreg via parallel factor (PARAFAC) analysis of fluorescence excitation-emission matrix (EEM). Chem Eng J, 2016, 299: 167-176. DOI:10.1016/j.cej.2016.04.020 |
| [99] | Huang X, Zheng JL, Tian SH, et al. Higher temperatures do not always achieve better antibiotic resistance gene removal in anaerobic digestion of swine manure. Appl Environ Microbiol, 2019, 85(7). |
| [100] | Ma SS, Fang C, Sun XX, et al. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour Technol, 2018, 259: 221-227. DOI:10.1016/j.biortech.2018.03.054 |
| [101] | Mohring SAI, Strzysch I, Fernandes MR, et al. Degradation and elimination of various sulfonamides during anaerobic fermentation: a promising step on the way to sustainable pharmacy?. Environ Sci Technol, 2009, 43(7): 2569-2574. DOI:10.1021/es802042d |
| [102] | Yan WF, Xiao Y, Yan WD, et al. The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: a review. Chem Eng J, 2019, 358: 1421-1437. DOI:10.1016/j.cej.2018.10.128 |
| [103] | Harnisch F, Gimkiewicz C, Bogunovic B, et al. On the removal of sulfonamides using microbial bioelectrochemical systems. Electrochem Commun, 2013, 26: 77-80. DOI:10.1016/j.elecom.2012.10.015 |
| [104] | Guo N, Wang YK, Yan L, et al. Effect of bio-electrochemical system on the fate and proliferation of chloramphenicol resistance genes during the treatment of chloramphenicol wastewater. Water Res, 2017, 117: 95-101. DOI:10.1016/j.watres.2017.03.058 |
| [105] | Wang L, You LX, Zhang JM, et al. Biodegradation of sulfadiazine in microbial fuel cells: reaction mechanism, biotoxicity removal and the correlation with reactor microbes. J Hazard Mater, 2018, 360: 402-411. DOI:10.1016/j.jhazmat.2018.08.021 |
| [106] | Wang L, Liu YL, Ma J, et al. Rapid degradation of sulphamethoxazole and the further transformation of 3-amino-5-methylisoxazole in a microbial fuel cell. Water Res, 2016, 88: 322-328. DOI:10.1016/j.watres.2015.10.030 |
| [107] | Zhang S, Song HL, Yang XL, et al. Dynamics of antibiotic resistance genes in microbial fuel cell-coupled constructed wetlands treating antibiotic-polluted water. Chemosphere, 2017, 178: 548-555. DOI:10.1016/j.chemosphere.2017.03.088 |
| [108] | Song HL, Li H, Zhang S, et al. Fate of sulfadiazine and its corresponding resistance genes in up-flow microbial fuel cell coupled constructed wetlands: effects of circuit operation mode and hydraulic retention time. Chem Eng J, 2018, 350: 920-929. DOI:10.1016/j.cej.2018.06.035 |
| [109] | Ding R, Yan WF, Wu Y, et al. Light-excited photoelectrons coupled with bio-photocatalysis enhanced the degradation efficiency of oxytetracycline. Water Res, 2018, 143: 589-598. DOI:10.1016/j.watres.2018.06.068 |
| [110] | Liu Y, Guan YT, Gao BY, et al. Antioxidant responses and degradation of two antibiotic contaminants in Microcystis aeruginosa. Ecotoxicol Environ Saf, 2012, 86: 23-30. |
| [111] | Hom-Diaz A, Norvill ZN, Blánquez P, et al. Ciprofloxacin removal during secondary domestic wastewater treatment in high rate algal ponds. Chemosphere, 2017, 180: 33-41. DOI:10.1016/j.chemosphere.2017.03.125 |
| [112] | Yu Y, Zhou YY, Wang ZL, et al. Investigation of the removal mechanism of antibiotic ceftazidime by green algae and subsequent microbic impact assessment. Sci Rep, 2017, 7(1): 4168. DOI:10.1038/s41598-017-04128-3 |
| [113] | Du YX, Wang J, Li HT, et al. The dual function of the algal treatment: antibiotic elimination combined with CO2 fixation. Chemosphere, 2018, 211: 192-201. DOI:10.1016/j.chemosphere.2018.07.163 |
| [114] | Guo RX, Chen JQ. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem Eng J, 2015, 260: 550-556. DOI:10.1016/j.cej.2014.09.053 |
