

西藏农牧学院,西藏 林芝 860000
网络出版时间:2018-07-06
基金项目:西藏自治区自然科学基金(No. 2016ZR-NY-11),西藏农牧学院预防兽医学科建设项目(No. 2017),中央财政支持地方高校发展专项资金(No. 2016-397)资助
摘要: 戊型肝炎病毒(Hepatitis E Virus,HEV)感染是一个重要的全球公共卫生问题,而猪被认为是HEV的天然宿主。HEV可以跨种间传播,且已经证实生吃感染的猪肉会导致人感染。在中国西藏许多地区仍然有生吃猪肉、猪肝等的习惯,且不同种家畜混合饲养,极易造成HEV感染和传播。然而中国西藏地区猪HEV流行情况报道甚少。文中对中国西藏5个地区市(拉萨、日喀则、山南、那曲和昌都)猪血清进行HEV Immunoglobulin-M (IgM)和IgG抗体检测,并通过逆转录巢氏PCR (RT-nPCR)进行HEV RNA检测和定量RT-PCR (qRT-PCR)进行病毒拷贝计算,首次报道了藏猪血清HEV RNA阳性率。结果显示,在西藏猪中HEV有较高的流行趋势。猪血清HEV IgM抗体阳性率高达7.6% (26/340),HEV IgG抗体阳性率为1.8% (6/340),HEV RNA阳性率高达7.6% (26/340),血清中病毒拷贝高达1.7×107 copies/mL,而且5个地区有不同的流行趋势。结果表明西藏猪HEV感染情况严重。有关部门应加强管理,以避免人与动物之间的交叉感染和暴发。
关键词: 戊型肝炎病毒 猪 中国西藏地区
Epidemiological investigation of hepatitis E virus infection in Tibetan swine population
Gongga, Yifei Wang, Yixicuomu, Qiongda, Suolangsizhu


Animal Science College, Tibet Agriculture & Animal Husbandry University, Linzhi 860000, Tibet, China
Received: May 7, 2018; Accepted: June 13, 2018
Supported by: Natural Science Foundation of Tibet Autonomous Region to Gong Ga, China (No. 2016ZR-NY-11), Funding for Preventive Veterinary Research in Tibet Agriculture & Animal Husbandry University (No. 2017), Central Government Supports Special Funds for the Development of Local Colleges and Universities in China (No. 2016-397)
Corresponding author:Suolangsizhu. Tel: +86-894-5822924; E-mail: xzslsz@163.com
Abstract: Hepatitis E virus (HEV) infection is a main global public health issue. HEV can be zoonotically transmitted across species, and swine is recognized as a major reservoir of HEV. However, information is lacking on the prevalence of HEV infection in Tibet of China, where raw pork and mixed farming of different species of domestic animals are consumed traditionally. In this study, swine serum was collected for HEV IgM and IgG antibodies test from five regions in Tibet of China. Meanwhile, HEV RNA was detected in swine sera. HEV has a high prevalence trend in Tibetan swine. Swine serum anti-HEV IgM antibody positive rate was as high as 7.6%, the positive rate of anti-HEV IgG antibody was 1.8%, the positive rate of HEV RNA also was 7.6%, the virus titers in serum was above 1.7×107 copies/mL, and there were different epidemic trends in five regions. In conclusion, antibody detection and RNA detection showed that swine in Tibet had a higher incidence of HEV infection. HEV infection in Tibetan swine is more serious and management should be strengthened to avoid cross-infection between humans and animals and outbreaks in Tibet.
Keywords: Hepatitis E virus swine Tibet (China)
Hepatitis E virus (HEV) is a positive-sense, single-stranded RNA virus, which is the sole member of the Orthohepevirus genus in the Hepeviridae family[1-2]. The World Health Organization has been reported that approximately 20 million people are infected by HEV every year worldwide[3] and caused the high mortality rate of approximately 20% in pregnant women[1, 4]. Moreover HEV also can cause chronic infection in immunocompromised people[5-6]. Recently, HEV has emerged as a global public health issue[7-8]. The main transmission route of the infection was fecal-oral route[9-10], food-chain and contaminated water are the main reason for the outbreak[11-14]. Besides, sporadic HEV infection is primarily caused by consumption of HEV contaminated meat in industrialized countries[15].
HEV mainly exists withseven genotypes (Gt1–7) and one serotype[2, 16-17]. Gt1 and Gt2 areonly infected to humans and lead to large outbreaks in developing regions[3, 18-19].Gt3 and Gt4 can be transmitted zoonotically through the ingestion of infectedmeat and cause infections in worldwide[10, 20]. The Gt5 and Gt6 areisolated from wild boars in Japan[21-22]. Gt7 is isolated from camelin Japan, which also can be transmitted to humans[23]. And othergenotypes have been found in several animal species, including rat (HEV C1)[24-25], ferret (HEV C2)[26], chicken (avian HEV)[27], bat (batHEV)[28] and trout (trout HEV)[29].
Swine are recognized asthe main natural reservoirs of HEV[30-31], and many researchhas reported that the HEV infection is highly prevalent in swine in many countries[32-33], including China[1, 34-37]. Tibet Province is located in southwestern China, where mixed farming of domestic animals is a common practice. But the prevalence of HEV in swine was rarely reported in Tibet, and the only one report being restricted to serological surveys[36].In this study, we aimed to assess the prevalence of HEV in Tibet swine bydetecting anti-HEV IgG and IgM in Tibetan swine serum. At the same time, we arethe first to report the positive rate of HEV RNA in Tibetan swine serum.
1 Materials and methods1.1 Sample collectionSerum samples (n=340, age=1±0.2 years old, male, n=209, female, n=131)were collected from the five cities from November 2016 to Mary 2017, includingShigatse city (n=140, male, n=87, female, n=53), Lhasacity (n=55, male, n=38, female, n=17), Lhoka city (n=54, male, n=35, female, n=19), Chamdo city (n=51, male, n=39, female, n=12), Nakchu city (n=40, male, n=28, female, n=12).The samples were stored at –40 ℃ until use.
1.2 Detection of anti-HEV IgG and IgM antibodiesSerum samples were testedfor the presence of anti-HEV IgG and IgM antibodies using commercialenzyme-linked immunosorbent assay kits (WanTai Beijing, China) containingrecombinantORF2 peptides from the HEV genome as well as both positive and negativecontrols. Samples were tested in duplicate according to the manufacturer’sinstructions, with cut off values for IgG and IgM assays set at 0.22 and 0.24, respectively, which were determined based on the mean optical density 450values from the negative controls.
1.3 Detection of HEV RNA in serumTotal RNA was extractedfrom the serum using the AxyPrepTM Body Fluid Viral DNA/RNA Miniprep Kit(Jiangsu, China) according to the manufacturer’s instructions.Reverse-transcription was performed using a reverse transcriptase kit (AMVXLfor real-time polymerase chain reaction RT-PCR; TaKaRa, Japan) according to themanufacturer’s directions. A 348-nucleotide amplicon from HEV open readingframe 2 (ORF2) was amplified by nested RT-PCR as previously described[1, 32].
1.4 Viraltiter in serumTheviral titer of HEV in sample was quantified using SYBR green-based quantitativeRT-PCR (qRT-PCR) with the specific primers as previously described[1, 32].In brief, 200 μL serum was subjected to RNA isolation. Isolated RNA was used tosynthesize the first-strand cDNA, and cDNA was added as a template for qRT-PCR.qRT-PCR was performed under the following conditions: 95 ℃ for 30 s, followedby 39 cycles of 95 ℃ for 5 s and 60 ℃ for 31 s. The procedure was conductedusing the BIO-RAD CFX Connect Real-Time System.
1.5 Statistical analysisPrism software (GraphPadSoftware) was used for statistical analysis. Comparisons between two groupswere performed with Wilcoxon matched pairs test. Differences were consideredsignificant at a P-value less than 0.05.
2 Results2.1 Seroprevalence of HEV in swine in Tibet of ChinaTo investigate theseroprevalence of HEV in Tibetan swine, we collected 340 serums from fivecities in Tibet (China) to detect the anti-HEV antibody. To our surprise, 26out of 340 (7.6%) serum sample were positive to anti-HEV IgM antibody, and theLhoka city has highest positive rate of anti-HEV IgM antibody up to 18.5%(10/54), Shigatse city was 5.0% (7/140), Lhasa city was 3.6% (2/55), Chamdocity was 5.9% (3/51) and Nakchu city was 10.0% (4/40), which were higher than the seroprevalence of anti-HEV IgM antibody in pigs in Shandongprovince of China (1.6%, 16/980) in 2014[38] and was lower than in Yunnan provinceof China (9.1%, 1/11) in 2009[39], but similar to that in Bavaria, Germany (7.0%, 36/516) in 2012. The difference analysis showed that thepositive rate of anti-IgM antibody in Lhoka city was significantly differentfrom the other three cities (Shigatse, Lhasa and Chamdo) (P < 0.05).But there was no significant difference between other cities (P > 0.05) (Fig. 1A).
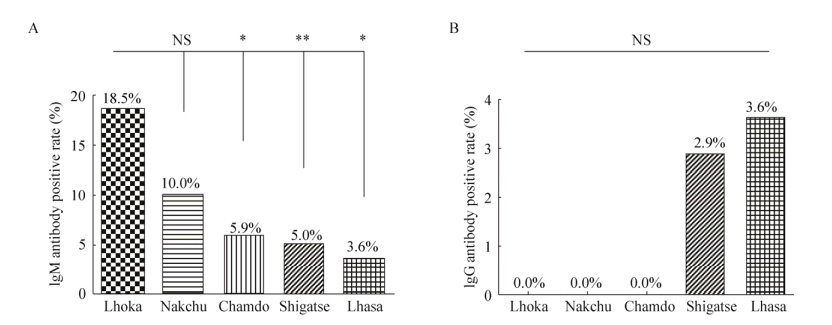 |
| Figure 1 Seroprevalence of HEV in Tibetan pigs of five cities in Tibet, China. (A) The positive rate of anti-HEV IgM in serum of Tibetan swine. (B) The positive rate of anti-HEV IgG in serum of Tibetan pigs. NS (not significant), P > 0.05, *P < 0.05, **P < 0.01. |
| 图选项 |
And 6 out of 340 (1.8%, 6/340) serum sample were positive toanti-HEV IgG antibody, the Lhasa city has highest positive rate of anti-HEV IgGantibody up to 3.6% (2/55), and the Shigatse city was 2.9% (4/140), but the Lhoka, Nakchu and Chamdocities were not tested in this research, which was lower than theseroprevalence of anti-HEV IgG antibody in pigs in Shandong province of China (66.4%, 651/980) in 2014[38], in Yunnan province, China (78.9%, 490/621) in2011[39], in Tibet, China (42.4%, 92/453) in 2015 and in Punjab, India (60.5%) in 2017[40]. The difference analysis showed that therewas no significant difference in the serum prevalence rate of anti-HEV IgGantibody between different five cities (P > 0.05) (Fig. 1B).
2.2 High prevalence of HEV infection in swine in Tibet of ChinaUp to date, there is noreport about the positive rate of HEV RNA in Tibetan swine serum. In thisstudy, the swine serum HEV RNA was detected by RT-nPCR (Fig. 2A). And we foundthat 26 out of 340 (7.6%, 26/340) serum was positive to HEV RNA, those positivesamples also were all the positive of anti-HEV IgM antibody, which was lowerthan Yunnan province of China (9.1%, 1/11) in 2011[39]and in South Brazil (20.0%) in 2017[41], but similar to that inPunjab, India (8.7%) in 2017[40].
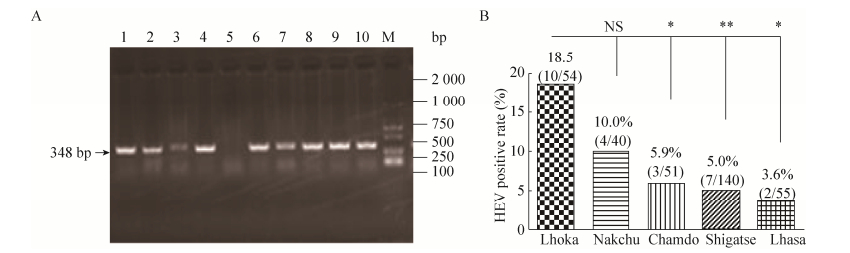 |
| Figure 2 Prevalence of HEV infection in Tibetan swine of five cities in Tibet, China. (A) The serum sample HEV RNA was detected by RT-nPCR. Lanes 1–10 is part of the sample number. M, DNA ladder. (B) The positive rate of HEV RNA in serum samples of Tibetan pigs of five cities in Tibet, China. NS (not significant). P > 0.05, *P < 0.05, **P < 0.01. |
| 图选项 |
And the five cities havedifferent HEV RNA positive rate, the Lhoka city has highest positive rate ofHEV RNA up to 18.5% (10/54), Nakchu city was 10.0% (4/40), Chamdo city was 5.9%(3/51), Shigatse city was 5% (7/140) and Lhasa city was 3.6% (2/55). Thedifference analysis showed that the positive rate of HEV RNA in Lhoka city wassignificantly different from the other three cities (Shigatse, Lhasa andChamdo) (P < 0.05). However, there was no significant differencebetween other cities (P > 0.05) (Fig. 2B).
2.3 The HEV viral titers in serum of swine in Tibet of ChinaTheviral titers in positive serums (n=26) were quantified by qRT-PCR. Theviral titers of HEV in serum ranged from 5.5×105 copies/mL to 1.7×107copies/mL.The high HEV titers in serum of swine indicate the high risk of HEV infectionin swine in Tibet of China (Fig. 3).
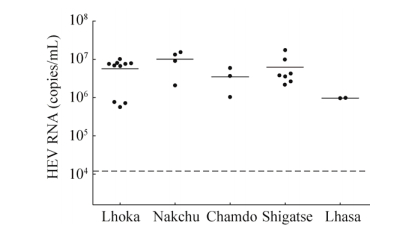 |
| Figure 3 The HEV viral titers in serum of Tibetan pigs of five cities in Tibet, China. Quantification of HEV genomic RNA from serum samples of Tibetan pigs. Unit for serum samples: copies/mL. |
| 图选项 |
3 DiscussionZoonotic transmission of HEV has caused a majorpotential public health problem in the world [9-10]. In this paper, we focusedon the Seroprevalence of HEV in swine in Tibet of China and we found the highlypositive rate (7.6%) of anti-HEV IgM antibody in swine in Tibet of China. Thepositive rate of anti-HEV IgM antibody in different regions was different, andthe difference analysis showed that the positive rate of anti-HEV IgM antibodyin Lhoka region was significantly higher in other three cities. And we alsofound the highly positive rate (1.8%) of anti-HEV IgG antibody in swine inTibet of China. The positive rate of anti-HEV IgG antibody in different regionswas also different. These results showed that the HEV infected in swine wasdifferent in Tibet, which may be related the local elevation, feeding methods and the environment.And we also reported high positive rate of HEV RNA (7.6%, 26/340) in Tibetanswine serum, which is the first report in Tibet of China. We found thatanti-HEV IgM antibody and HEV RNA positive rate were higher in this study, anddifferent regions of swine serum had high virus copies, which showed that pigsin Tibet are in the acute phase of infection and with the risk of outbreak inTibet. So management should be strengthened to avoid cross-infection andoutbreaks.
Compared with the previousstudies[36, 39-41], the high positive rate of anti-HEV IgM antibodyand HEV RNA were found in serum in Tibetan swine, but the positive rate ofanti-HEV IgG antibody was low. At the same time, the positive rate in differentcities were divergent, which may be attributed to the different elevation, feeding ways of Tibetan swine and climate. However, compared with the resultsof swine HEV infection in Tibet reported in 2017 by Li et al[36], our survey involves more regions but relatively fewer samples in each region, which may explain why our positive rate is lower than Li et al. In order tofurther investigate the epidemic situation of HEV in swine in Tibet of China, we will expand sampling areas and sampling quantities in the next research.
Consuming raw (oruncooked) or undercooked meat has been confirmed to beassociated with HEV infection[42-43], more importantly, consuming rawpork meat, liver remain widespread in Tibet of China, which may increase therisk of HEV infected. In addition, it has been reported that veterinarianclosely contacted with swine was found with a higher anti-HEV IgG antibodytiters[44], and mixed farming of different species of domesticanimals also caused the contact infection. However, due to various factors, such as conditions and economics, the different species of domestic animals arestill in mixed farming and grazing in Tibet of China, which also increases therisk of cross-infection between animals and humans and that may be the reasonwhy the swine has high positive rate of anti-HEV IgM antibody and HEV RNA inTibet of China.
4 ConclusionsIn summary, high anti-HEV IgM and IgG antibody positive rate was detected in Tibetan swineserum. In addition, high HEV RNA positive rate and high HEV viral titers werealso detected to prove that Tibetan swine are being infected with HEV. Managementshould be strengthened to avoid cross-infection between humans and animals inTibet of China.
REFERENCES
| [1] | Huang F, Li YL, Yu WH, et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology, 2016, 64(2): 350-359. DOI:10.1002/hep.28668 |
| [2] | Nimgaonkar I, Ding Q, Schwartz RE, et al. Hepatitis E virus: advances and challenges. Nat Rev Gastroenterol Hepatol, 2018, 15(2): 96-110. |
| [3] | Rein DB, Stevens GA, Theaker J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology, 2012, 55(4): 988-997. DOI:10.1002/hep.25505 |
| [4] | Bernuau J, Nicand E, Durand F. Hepatitis E-associated acute liver failure in pregnancy: an Indian puzzle. Hepatology, 2008, 48(5): 1380-1382. DOI:10.1002/hep.22619 |
| [5] | Peron JM, Mansuy JM, Recher C, et al. Prolonged hepatitis E in an immunocompromised patient. J Gastroenterol Hepatol, 2006, 21(7): 1223-1224. DOI:10.1111/jgh.2006.21.issue-7 |
| [6] | Singh A, Seth R, Gupta A, et al. Chronic hepatitis E-an emerging disease in an immunocompromised host. Gastroenterol Rep, 2018, 6(2): 152-155. |
| [7] | Spahr C, Knauf-Witzens T, Vahlenkamp T, et al. Hepatitis E virus and related viruses in wild, domestic and zoo animals: a review. Zoonoses Public Hlth, 2018, 65(1): 11-29. DOI:10.1111/zph.12405 |
| [8] | Nan YC, Wu CY, Zhao Q, et al. Zoonotic hepatitis E virus: an ignored risk for public health. Front Microbiol, 2017, 8: 2396. DOI:10.3389/fmicb.2017.02396 |
| [9] | Kamar N, Izopet J, Pavio N, et al. Hepatitis E virus infection. Nat Rev Dis Prim, 2017, 3: 17086. DOI:10.1038/nrdp.2017.86 |
| [10] | Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet (London, England), 2012, 379(9835): 2477-2488. DOI:10.1016/S0140-6736(11)61849-7 |
| [11] | Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res, 2011, 161(1): 23-30. DOI:10.1016/j.virusres.2011.01.016 |
| [12] | Colson P, Borentain P, Queyriaux B, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis, 2010, 202(6): 825-834. DOI:10.1086/653018 |
| [13] | Cossaboom CM, Heffron CL, Cao DJ, et al. Risk factors and sources of foodborne hepatitis E virus infection in the United States. J Med Virol, 2016, 88(9): 1641-1645. DOI:10.1002/jmv.v88.9 |
| [14] | Aggarwal R, Naik SR. Hepatitis E: intrafamilial transmission versus waterborne spread. J Hepatol, 1994, 21(5): 718-723. DOI:10.1016/S0168-8278(94)80229-7 |
| [15] | Takahashi M, Okamoto H. Features of hepatitis E virus infection in humans and animals in Japan. Hepatol Res, 2014, 44(1): 43-58. DOI:10.1111/hepr.2014.44.issue-1 |
| [16] | Smith JL. A review of hepatitis E virus. J Food Prot, 2001, 64(4): 572-586. DOI:10.4315/0362-028X-64.4.572 |
| [17] | Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol, 2003, 13(3): 145-154. DOI:10.1002/(ISSN)1099-1654 |
| [18] | de Alencar Arrais Guerra JA, Kampa KC, Morsoletto DGB, et al. Hepatitis E: a literature review. J Clin Trans Hepatol, 2017, 5(4): 376-383. |
| [19] | Huang CC, Nguyen D, Fernandez J, et al. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology, 1992, 191(2): 550-558. DOI:10.1016/0042-6822(92)90230-M |
| [20] | Murali AR, Kotwal V, Chawla S. Chronic hepatitis E: a brief review. World J Hepatol, 2015, 7(19): 2194-2201. DOI:10.4254/wjh.v7.i19.2194 |
| [21] | Takahashi M, Nishizawa T, Sato H, et al. Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J Gen Virol, 2011, 92(Pt 4): 902-908. |
| [22] | Smith DB, Simmonds P, Jameel S, et al. Consensus proposals for classification of the family Hepeviridae. J Gen Virol, 2015, 96(Pt 5): 1191-1192. |
| [23] | Lee GH, Tan BH, Teo ECY, et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology, 2016, 150(2): 355-357. DOI:10.1053/j.gastro.2015.10.048 |
| [24] | Obana S, Shimizu K, Yoshimatsu K, et al. Epizootiological study of rodent-borne hepatitis E virus HEV-C1 in small mammals in Hanoi, Vietnam. J Veter Med Sci, 2017, 79(1): 76-81. DOI:10.1292/jvms.16-0355 |
| [25] | Johne R, Dremsek P, Kindler E, et al. Rat hepatitis E virus: geographical clustering within Germany and serological detection in wild Norway rats (Rattus norvegicus). Infect, Genet Evolut, 2012, 12(5): 947-956. DOI:10.1016/j.meegid.2012.02.021 |
| [26] | Raj VS, Smits SL, Pas SD, et al. Novel hepatitis E virus in ferrets, the Netherlands. Emerg Infect Dis, 2012, 18(8): 1369-1370. DOI:10.3201/eid1808.111659 |
| [27] | Huang FF, Sun ZF, Emerson SU, et al. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol, 2004, 85(Pt 6): 1609-1618. |
| [28] | Drexler JF, Seelen A, Corman VM, et al. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J Virol, 2012, 86(17): 9134-9147. DOI:10.1128/JVI.00800-12 |
| [29] | Batts W, Yun SS, Hedrick R, et al. A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res, 2011, 158(1/2): 116-123. |
| [30] | La RG, Libera SD, Brambilla M, et al. Hepatitis E virus (Genotype 3) in slurry samples from swine farming activities in Italy. Food Environ Virol, 2017, 9(2): 219-229. DOI:10.1007/s12560-016-9270-4 |
| [31] | Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA, 1997, 94(18): 9860-9865. DOI:10.1073/pnas.94.18.9860 |
| [32] | Huang FF, Haqshenas G, Guenette DK, et al. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J Clin Microbiol, 2002, 40(4): 1326-1332. DOI:10.1128/JCM.40.4.1326-1332.2002 |
| [33] | Intharasongkroh D, Sa-Nguanmoo P, Tuanthap S, et al. Hepatitis E virus in pork and variety meats sold in fresh markets. Food Environ Virol, 2017, 9(1): 45-53. DOI:10.1007/s12560-016-9258-0 |
| [34] | Long FY, Yu WH, Yang CC, et al. High prevalence of hepatitis E virus infection in goats. J Med Virol, 2017, 89(11): 1981-1987. DOI:10.1002/jmv.v89.11 |
| [35] | Shuai JB, Li LH, Li AY, et al. Full genome analysis of swine genotype 3 hepatitis E virus isolated from eastern China. J Zhejiang Univ Sci B, 2017, 18(6): 549-554. DOI:10.1631/jzus.B1600419 |
| [36] | Zhang LH, Li K, Huang SC, et al. Seroprevalence and risk factors associated with hepatitis E virus infections among people and pigs in Tibet, China. Acta Trop, 2017, 172: 102-106. DOI:10.1016/j.actatropica.2017.04.033 |
| [37] | Jia ZY, Yi Y, Liu JY, et al. Epidemiology of hepatitis E virus in China: results from the Third National Viral Hepatitis Prevalence Survey, 2005-2006. PLoS ONE, 2014, 9(10): e110837. DOI:10.1371/journal.pone.0110837 |
| [38] | Wang XJ, Zhao Q, Jiang FL, et al. Genetic characterization and serological prevalence of swine hepatitis E virus in Shandong province, China. Veter Microbiol, 2014, 172(3/4): 415-424. |
| [39] | Li WG, Shu XH, Pu YL, et al. Seroprevalence and molecular detection of hepatitis E virus in Yunnan province, China. Arch Virol, 2011, 156(11): 1989-1995. DOI:10.1007/s00705-011-1089-6 |
| [40] | Bansal M, Kaur S, Deka D, et al. Seroepidemiology and molecular characterization of hepatitis E virus infection in swine and occupationally exposed workers in Punjab, India. Zoon Public Health, 2017, 64(8): 662-672. DOI:10.1111/zph.12363 |
| [41] | Passos-Castilho AM, Granato CFH. High frequency of hepatitis E virus infection in swine from South Brazil and close similarity to human HEV isolates. Brazil J Microbiol, 2017, 48(2): 373-379. DOI:10.1016/j.bjm.2016.10.022 |
| [42] | Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol, 2003, 84(Pt 9): 2351-2357. |
| [43] | Hijioka S, Sato Y, Iwashita Y, et al. A case of acute hepatitis E who had a history of frequent ingestion of raw meat and viscera from wild deer and boars. Nihon Shokakibyo Gakkai Zasshi, 2005, 102(6): 723-728. |
| [44] | Meng XJ, Wiseman B, Elvinger F, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol, 2002, 40(1): 117-122. DOI:10.1128/JCM.40.1.117-122.2002 |
